Non-celiac wheat sensitivity (NCWS) is a clinical entity induced by the ingestion of gluten that leads to intestinal and/or extraintestinal symptoms, and is diagnosed when celiac disease and wheat allergy have been ruled out. In addition to gluten, other grains’ components, including amylase trypsin inhibitors (ATIs) and fermentable short-chain carbohydrates (FODMAPs), may trigger symptoms in NCWS subjects. Several studies suggest that, compared with tetraploid and hexaploid modern wheats, ancient diploid wheats species could possess a lower immunogenicity for subjects suffering from NCWS.
- non celiac wheat sensitivity
- diploid wheat
- common wheat
- amylase trypsin inhibitor
- FODMAP
1. Introduction
2. Mucosal Immune Responses in NCWS
| Gut Immune Activation in NCWS | References |
|---|---|
Innate immune response
|
|
Adaptiveimmune response
|
|
Autoantibodies
|
|
Intestinal permeability
|
|
Mucosal immune cells
|
|
3. Gluten Components
Researchers have previously investigated, in in vitro models, the immunological properties of gliadin protein from two monococcum cvs, Monlis and Norberto-ID331, in view of their possible use in CD patients [40]. Researchers found that partially digested gliadin proteins extracted from two monococcum lines, Monlis and Norberto-ID331, induced adaptive immune response in CD patients, whereas the innate immune response could be elicited only by gliadin from Monlis cv [40]. Subsequently, researchers have demonstrated, by proteomic analysis, that almost all immunotoxic gluten peptides from Monlis and Norberto-ID331, are in vitro degraded during digestion by gastric-duodenal and brush border membrane (GD-BBM) enzymes, whereas gluten immunogenic peptides from hexaploid Triticum aestivum resist intestinal [41] . Clinical trials have shown that T. monococcum is toxic for CD patients, but it was well tolerated by the majority of patients [42], suggesting a potential effcacy in patients suffering from other gluten-related disorders, such as NCWS.
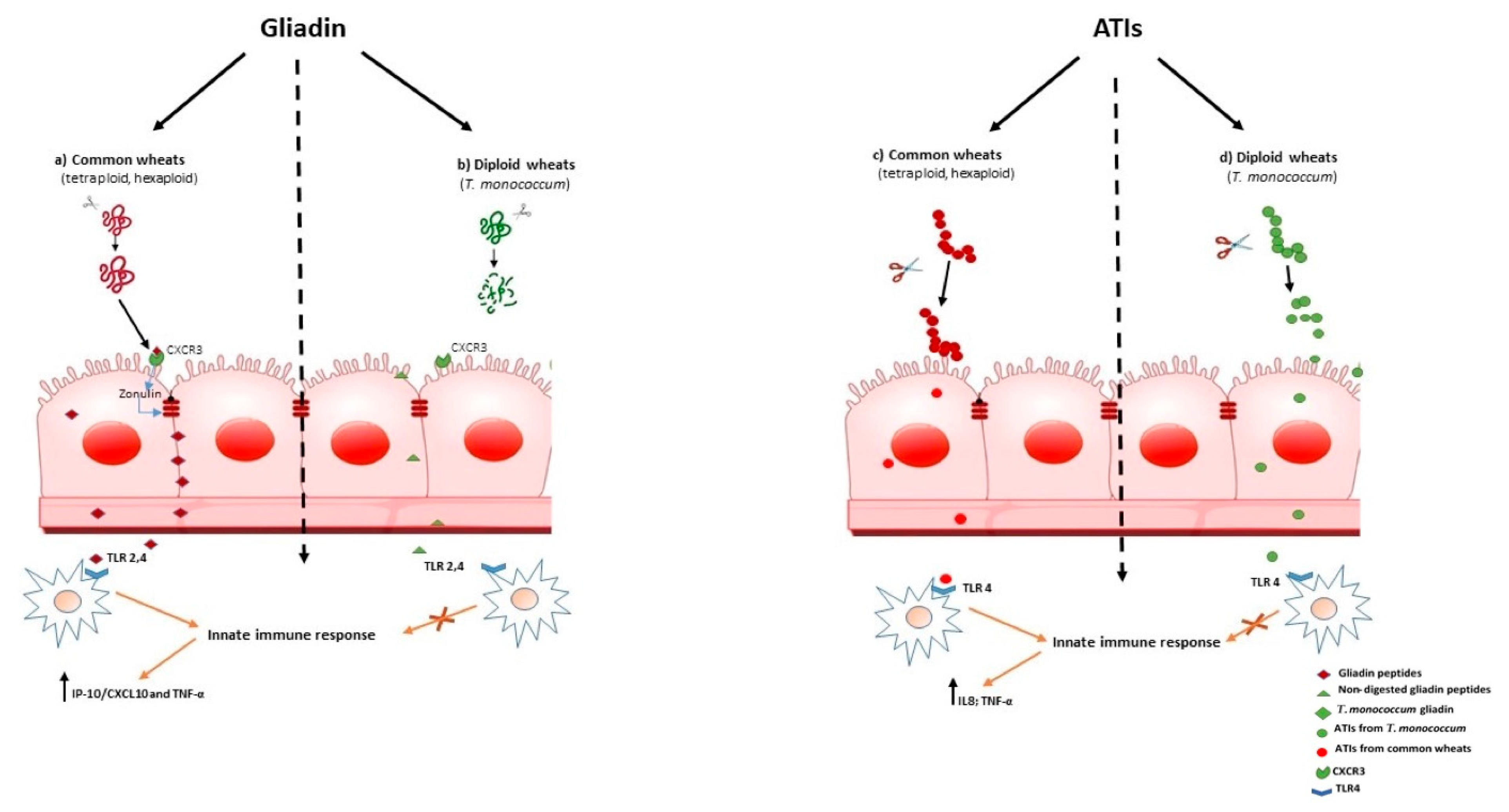
Increased intestinal permeability in patients suffering fron wheat-related disorders, could be an early events that precedes the onset of gut immune activation. In CD it was shown that myeloid differentiation factor 88 (MyD88), a key adapter molecule in the TLR/IL-1R signaling pathways, induces release of zonulin, a mediator of gut permeability, upon non-digested gliadin binding to CXCR3 on enterocytes, as a result inducing greater epithelial permeability and subsequent paracellular gliadin passage to the gut mucosa [43] (Figure 1a). These data support the model for the innate immune response to gliadin in the initiation of CD. Similar mechanisms may also underlie the increased intestinal permeability reported in NCWS. As researchers have shown that T. monococcum gliadin had a marked susceptibility to gastrointestinal digestion, researchers can hypothesize that such mechanism, triggered by non-digested gliadin of common wheats, may not be elicited (Figure 1, b). Thus, the innate immune response could be prevented.
4. Non-gluten components
The non-gluten wheat proteins comprise a mixture of components with), such as ATIs, which may contribute to defence plant from pests and parasites.
To date, ATIs have been shown to be potent activators of the innate immune system response, engaging TLR-4 with release of proinflammatory cytokines in myeloid cells (IL-8 and IL-12), of both patients with CD and non-diseased controls. For their general TLR4 stimulatory activity, ATIs were suspected to have pathogenic roles in patients with wheat-related hypersensitivities such as NCWS or irritable bowel syndrome (IBS) [5][44][45]
Interestingly, it was found that modern wheat contains high concentrations of ATIs, compared with ancient diploid wheat [5] [46] [47],[48]. Therefore, considering the pro-inflammatory effect of ATIs, T. monococcum wheat could retains a reduced immunostimulating activity for subjects suffering from wheat related diseases.
Zevallos and co-workers found that older wheat variants, as T. monococcum, had lower bioactivity than modern wheat [13];. More recently, researchers found that PC-digested ATIs purified from Triticum aestivum induced IL-8 and TNF-α secretion in organ culture of jejunal mucosa of treated CD patients, whereas the capability of ATIs from T. monococcum to stimulate innate immunity was meaningfully affected [49]. It has been reported that the resistance to gastrointestinal digestion is an important constrain in determining the immune stimulatory and toxicity properties of gliadin peptides[50] . Therefore, researchers' data suggest that the susceptible to enzymatic hydrolysis of ATIs from diploid T. monococcum resulted in a failure to induce the innate immune response (Figure 1, d). In contrast, the stability to hydrolysis by human digestive enzymes of ATIs from hexaploid wheat, affects the activation of mucosal innate immune response (Figure 1, c).
Therefore, researchers can hypothesize that a regular diet based on T. monococcum in NCWS patients, might prevent gut immune activation.
5. Beyond immunogenicity: nutritional features of diploid wheats
The increasing attention to the nutritional aspects of food has led to the search for alternatives to the traditional T. aestivum wheat.
Compared to modern wheats, diploid wheats showed a better nutritional quality and relevant potential for human consumption. In particular, ancient wheats contains higher levels of antioxidant compounds as α- and β-carotenes, lutein, zeaxanthin, tocols, conjugated polyphenols, alkyl resorcinols and phytosterols, retinol, phosphorus, potassium, riboflavin and pyridoxine [51][52][53][54][55].
Moreover, ancient wheats had a lower quantity of dietary fibre and carbohydrate but a higher content of proteins, lipids (mostly unsaturated fatty acids), fructans, thiamine and a number of other B vitamins, zinc and iron [52][53][54][55] [56] , compared to modern wheats, that gives them properties useful in preventing some pathological conditions.Ancient wheats are still cultivated nowaday only in some areas of the world, including France, Germany, Austria, Hungary, Bulgaria and Italy, but the increasing interest to healthier foods has increased the popularity of their use and, consequently, has caused an increase in their production.For all these features the use of ancient wheats may become more relevant in human consumption, especially in the development of new or special functional foods with superior nutritional quality.
6. Conclusion
Gluten, and other wheat proteins, including ATIs and FODMAPs, have been identified as possible factors for generation of intestinal and extra-intestinal symptoms in subjects suffering from wheat-related disorders, such as NCWS. In particular, it is well-known that gluten and ATIs possess immune stimulating activity. Therefore, dietary exposure to the combination of gluten and ATIs exacerbates intestinal immune dysregulation and increase risk to develop wheat-related disorders. T. monococcum, the oldest and most primitive cultivated wheat, unexposed to genetic improvements, has been suggested to possibly exert a reduced immunostimulating activity compared to common wheats and, consequently, embodies the role of a fitting candidate to be introduced into the diet of such patients. Therefore, clinical studies on NCWS patients to assess the effects of a TM wheat–based foods diet are warranted.
This entry is adapted from the peer-reviewed paper 10.3390/cells11152389
References
- Wieser, H.; Koehler, P.; Scherf, K.A. The Two Faces of Wheat. Front. Nutr. 2020, 7, 517313.
- Cabanillas, B. Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit Rev. Food Sci Nutr. 2020, 60, 2606–2621.
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977.
- Gasbarrini, G.; Mangiola, F. Wheat-related disorders: A broad spectrum of ‘evolving’ diseases. United Eur. Gastroenterol. J. 2014, 2, 254–262.
- Aufiero, V.R.; Fasano, A.; Mazzarella, G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ From Celiac Disease. Mol. Nutr. Food Res. 2018, 62, e1700854.
- Igbinedion, S.O.; Ansari, J.; Vasikaran, A. Non-celiac gluten sensitivity: All wheat attack is not celiac. World J. Gastroenterol. 2017, 23, 7201–7210.
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23.
- Lammers, K.M.; Khandelwal, S.; Chaudhry, F.; Kryszak, D.; Puppa, E.L.; Casolaro, V.; Fasano, A. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 2011, 132, 432–440.
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408.
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911.e3.
- Di Liberto, D.; Mansueto, P.; D’Alcamo, A.; Lo Pizzo, M.; Lo Presti, E.; Geraci, G.; Fayer, F.; Guggino, G.; Iacono, G.; Dieli, F.; et al. Predominance of Type 1 Innate Lymphoid Cells in the Rectal Mucosa of Patients With Non-Celiac Wheat Sensitivity: Reversal After a Wheat-Free Diet. Clin. Transl. Gastroenterol. 2016, 7, e178.
- Caminero, A.; Galipeau, H.J.; McCarville, J.L.; Johnston, C.W.; Bernier, S.P.; Russell, A.K.; Jury, J.; Herran, A.R.; Casqueiro, J.; Tye-Din, J.A.; et al. Duodenal Bacteria From Patients with Celiac Disease and Healthy Subjects Distinctly Affect Gluten Breakdown and Immunogenicity. Gastroenterology 2016, 151, 670–683.
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113.
- Iacomino, G.; Rotondi Aufiero, V.; Di Stasio, L.; Picascia, S.; Iannaccone, N.; Giardullo, N.; Troncone, R.; Gianfrani, C.; Mamone, G.; Mazzarella, G. Triticum monococcum amylase trypsin inhibitors possess a reduced potential to elicit innate immune response in celiac patients compared to Triticum aestivum. Food Res. Int. 2021, 145, 110386.
- Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Figueroa-Salcido, O.G.; Ontiveros, N. Non-Celiac Gluten Sensitivity: An Update. Medicina 2021, 57, 526.
- Brottveit, M.; Beitnes, A.C.; Tollefsen, S.; Bratlie, J.E.; Jahnsen, F.L.; Johansen, F.E.; Sollid, L.M.; Lundin, K.E. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 2013, 108, 842–850.
- Mansueto, P.; Di Liberto, D.; Fayer, F.; Soresi, M.; Geraci, G.; Giannone, A.G.; Seidita, A.; D’Alcamo, A.; La Blasca, F.; Pizzo, M.L.; et al. TNF-α, IL-17 and IL-22 production in the rectal mucosa of non-celiac wheat sensitivity patients: Role of adaptive immunity. Am. J. Physiol. Liver Physiol. 2020, 319, G281–G288.
- Castillo-Rodal, A.I.; Furuzawa-Carballeda, J.; Peláez-Luna, M.; Castro-Gómez, J.; López-Vidal, Y.; Uscanga, L. More fuel to the fire: Some patients with non-celiac self-reported wheat sensitivity exhibit adaptive immunological responses in duodenal mucosa. BMC Gastroenterol. 2020, 20, 414.
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1907.
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685.
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937.
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576.
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020.
- Zanini, B.; Villanacci, V.; Marullo, M.; Cadei, M.; Lanzarotto, F.; Bozzola, A.; Ricci, C. Duodenal histological features in suspected non-celiac gluten sensitivity: New insights into a still undefined condition. Virchows Arch. 2018, 473, 229–234.
- Losurdo, G.; Piscitelli, D.; Pezzuto, F.; Fortarezza, F.; Covelli, C.; Marra, A.; Iannone, A.; Amoruso, A.; Principi, M.; Ierardi, E.; et al. T Helper Lymphocyte and Mast Cell Immunohistochemical Pattern in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Pract. 2017, 2017, 5023680.
- Giancola, F.; Volta, U.; Repossi, R.; Latorre, R.; Beeckmans, D.; Carbone, F.; Van den Houte, K.; Bianco, F.; Bonora, E.; Gori, A.; et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten/wheat sensitivity. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13814.
- Carroccio, A.; Giannone, G.; Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; Iacobucci, R.; Porcasi, R.; Catalano, T.; Geraci, G.; et al. Duodenal and Rectal Mucosa Inflammation in Patients With Non-celiac Wheat Sensitivity. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 682–690.
- Rostami, K.; Ensari, A.; Marsh, M.N.; Srivastava, A.; Villanacci, V.; Carroccio, A.; Aghdaei, H.A.; Bai, J.C.; Bassotti, G.; Becheanu, G.; et al. Gluten Induces Subtle Histological Changes in Duodenal Mucosa of Patients with Non-Coeliac Gluten Sensitivity: A Multicentre Study. Nutrients 2022, 14, 2487.
- Vilela, E.G.; de Abreu Ferrari, M.; de Gama Torres, H.O.; Martins, F.P.; Goulart, E.M.; Lima, A.S.; da Cunha, A.S. Intestinal permeability and antigliadin antibody test for monitoring adult patients with celiac disease. Dig. Dis. Sci. 2007, 52, 1304–1309.
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity: An Immune-Mediated Condition with Systemic Manifestations. Gastroenterol. Clin. 2019, 48, 165–182.
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81.
- Stamnaes, J.; Sollid, L.M. Celiac disease: Autoimmunity in response to food antigen. Semin Immunol. 2015, 27, 343–352.
- Sapone, A.; Lammers, K.M.; Mazzarella, G.; Mikhailenko, I.; Cartenì, M.; Casolaro, V.; Fasano, A. Differential mucosal IL-17 expression in two gliadin-induced disorders: Gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 2010, 152, 75–80.
- Abadie, V.; Jabri, B. Il-15: A Central Regulator of Celiac Disease Immunopathology. Immunol. Rev. 2014, 260, 221–234.
- Herrera, M.G.; Pizzuto, M.; Lonez, C.; Rott, K.; Hütten, A.; Sewald, N.; Ruysschaert, J.-M.; Dodero, V.I. Large Supramolecular Structures of 33-Mer Gliadin Peptide Activate Toll-like Receptors in Macrophages. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1417–1427.
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; van Veelen, P.; Drijfhout, J.W.; Jonker, H.; van Soest, L.; Smulders, M.J.; Bosch, D.; Gilissen, L.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806.
- Huang, S.; Sirikhachornkit, A.; Su, X.; Faris, J.; Gill, B.; Haselkorn, R.; Gornicki, P. Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc. Natl. Acad. Sci. USA 2002, 99, 8133–8138.
- Molberg, O.; Uhlen, A.K.; Jensen, T.; Flaete, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401.
- Wieser, H. Comparative investigations of gluten proteins from different wheat species. III. N-terminal amino acid sequences of α-gliadins potentially toxic for coeliac patients. Eur. Food Res. Technol. 2001, 213, 183–186.
- Gianfrani C., Maglio M., Rotondi Aufiero V., Camarca A., Vocca I., Iaquinto G., Giardullo N., Pogna N., Troncone R., Auricchio S., et al. Immunogenicity of monococcum wheat in celiac patients. Am. J. Clin. Nutr. 2012;96:1339–1345. doi: 10.3945/ajcn.112.040485. [PubMed] [CrossRef] [Google Scholar]
- Gianfrani C., Camarca A., Mazzarella G., Di Stasio L., Giardullo N., Ferranti P., Picariello G., Rotondi Aufiero V., Picascia S., Troncone R., et al. Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: Implication for celiac disease. Mol. Nutr. Food Res. 2015;59:1844–1854. doi: 10.1002/mnfr.201500126. [PubMed] [CrossRef] [Google Scholar]
- Zanini B., Villanacci V., De Leo L., Lanzini A. Triticum monococcum in patients with celiac disease: A phase II open study on safety of prolonged daily administration. Eur. J. Nutr. 2015;54:1027–1029. doi: 10.1007/s00394-015-0892-3. [PubMed] [CrossRef] [Google Scholar]
- Lammers K.M., Lu R., Brownley J., Lu B., Gerard C., Thomas K., Rallabhandi P., Shea-Donohue T., Tamiz A., Alkan S., et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Schuppan D., Zevallos V. Wheat amylase trypsin inhibitors as nutritional activators of innate immunity. Dig. Dis. 2015;33:260–263. doi: 10.1159/000371476. [PubMed] [CrossRef] [Google Scholar] , [CrossRef] [Google Scholar]
- Tilg H., Koch R., Moschen A.R. Proinflammatory Wheat Attacks on the Intestine: Alpha-Amylase Trypsin Inhibitors as New Players. Gastroenterology. 2013;144:1561–1563. doi: 10.1053/j.gastro.2013.04.035. [PubMed]
- Geisslitz S., Ludwig C., Scherf K.A., Koehler P. Targeted LC-MS/MS Reveals Similar Contents of α-Amylase/Trypsin-Inhibitors as Putative Triggers of Nonceliac Gluten Sensitivity in All Wheat Species except Einkorn. J. Agric. Food Chem. 2018;66:12395–12403. doi: 10.1021/acs.jafc.8b04411. [PubMed] [CrossRef] [Google Scholar]
- Zoccatelli G., Sega M., Bolla M., Cecconi D., Vaccino P., Rizzi C., Chignola R., Brandolini A. Expression of α-amylase inhibitors in diploid Triticum species. Food Chem. 2012;135:2643–2649. doi: 10.1016/j.foodchem.2012.06.123. [PubMed] [CrossRef] [Google Scholar]
- Geisslitz S., Longin C.F.H., Koehler P., Scherf K.A. Comparative quantitative LC-MS/MS analysis of 13 amylase/trypsin inhibitors in ancient and modern Triticum species. Sci. Rep. 2020;10:14570. doi: 10.1038/s41598-020-71413-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Caminero A., McCarville J.L., Zevallos V.F., Pigrau M., Yu X.B., Jury J., Galipeau H.J., Clarizio A.V., Casqueiro J., Murray J.A., et al. Lactobacilli Degrade Wheat Amylase Trypsin Inhibitors to Reduce Intestinal Dysfunction Induced by Immunogenic Wheat Proteins. Gastroenterology. 2019;156:2266–2280. doi: 10.1053/j.gastro.2019.02.028. [PubMed] [CrossRef] [Google Scholar]
- Shan L., Molberg O., Parrot I., Hausch F., Filiz F., Gray G., Sollid L., Koshla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [PubMed] [CrossRef] [Google Scholar ].
- Brandolini A., Hidalgo A., Gabriele S., Heun M. Chemical composition of wild and feral diploid wheats and their bearing on domesticated wheats. J. Cereal Sci. 2015;63:122–127. doi: 10.1016/j.jcs.2015.03.005. [CrossRef] [Google Scholar];
- Hidalgo A., Brandolini A. Nutritional properties of einkorn wheat (Triticum monococcum L.) J. Sci. Food Agric. 2014;94:601–612. doi: 10.1002/jsfa.6382. [PubMed] [CrossRef] [Google Scholar];
- Abdel-All E., Hucl P.J., Sosulski F.W. Compositional and nutritional characteristics of spring einkorn and spelt wheats. Cereal Chem. J. 1995;72:621–624. [Google Scholar];
- Hidalgo A., Alamprese C., Marti A., Galli S., Terno A.B., Brandolini A. Nutritional and technological properties of non-traditional einkorn (Triticum monococcum) wheat pasta. Lwt-Food Sci. Technol. 2020;133:109932. doi: 10.1016/j.lwt.2020.109932. [CrossRef] [Google Scholar];
- Velu G., Crespo Herrera L., Guzman C., Huerta J., Payne T., Singh R.P. Assessing Genetic Diversity to Breed Competi-tive Biofortified Wheat With Enhanced Grain Zn and Fe Concentrations. Front. Plant. Sci. 2019;9:1971. doi: 10.3389/fpls.2018.01971. [PMC free article] [PubMed] [CrossRef] [Google Scholar].
- Dinu M., Whittaker A., Pagliai G., Benedettelli S., Sofi F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018;52:1–9. doi: 10.1016/j.jnutbio.2017.09.001. [PubMed] [CrossRef] [Google Scholar]
