1. Introduction
The kidney disease improving global outcomes (KDIGO) initiative classifies an individual as having CKD if the abnormalities of kidney structure or function persist for over three months, although the definition is somewhat problematic in the elderly, in which some of the decline of kidney function may be considered to be a normal part of the aging process [
1]. The global burden of CKD has increased in recent years due to an aging global population and the rise of type 2 diabetes with diabetic nephropathy; moreover, the improved methods for early identification of CKD may also contribute to this [
2,
3,
4,
5,
6]. With more than 10% of the adult population being affected by CKD at present, it is projected to become the fifth leading cause of mortality worldwide by 2040 [
5]. The prevalence of patients with CKD progressing to kidney failure requiring kidney replacement therapy has increased, with registry data documenting a greater number of patients receiving dialysis worldwide; although universal access to dialysis is lacking in many developing countries. CKD therefore constitutes as an important global health issue [
7,
8].
The advanced stages of CKD involve a spectrum of secondary complications, such as anemia, secondary hyperparathyroidism/CKD-mineral bone disease (CKD-MBD), malnutrition, and electrolyte disturbances [
5,
9]. Together with a variety of symptoms that are seen in advanced CKD, they make up the clinical syndrome of uremia, which is typically seen in patients who are close to being initiated on dialysis. Pruritus is a common and often particularly bothersome symptom in uremia, with a considerable impact on patients’ symptom burden overall. The nomenclature has changed recently from “uremic pruritus” to CKD-associated pruritus (CKDaP) in order to account for the non-linear relationship between itch and the degree of uremia overall [
10]. CKDaP is associated with worsened overall patient outcomes and previous studies describe not only reduced health-related quality of life (HRQoL), but also increased sleep disturbance, poorer adherence to medication and dialysis and, importantly, increased rates of depression [
10,
11]. Patients with CKDaP are more likely to sustain skin infections and hospitalization and their mortality is high when compared to those without CKDaP [
10,
11].
The pathophysiology of CKDaP remains poorly understood, despite the fact that it is such a common and important feature of advanced kidney disease. CKDaP is perceived as a multifactorial process, which is attributed to a combination of local skin and systemic factors that are observed with advanced CKD. Progress has been made in terms of a more objective and structured assessment of CKDaP [
12,
13,
14,
15]. Establishing a consensus definition of CKDaP and creating patient-reported outcome- (PRO) oriented symptom severity scales are key features of this process [
16,
17]. Recent research has also focused on patient-specific goals of CKDaP treatment [
18]. Importantly, new options for treatment have become available very recently, particularly for the severe end of the disease spectrum [
19].
In this review, we provide an update regarding the epidemiology and pathophysiology of CKDaP. We also discuss current approaches to the assessment and the diagnosis and review the common and important differential diagnoses. Finally, we evaluate the various management strategies for CKDaP and highlight the gaps in our knowledge, as well as the avenues for future research. The study selection for this narrative review encompassed the input of the following search terms: “Pruritus”, “Chronic Kidney Disease-associated Pruritus”, “Epidemiology”, “Pathophysiology”, “Clinical Assessment”, “Differential Diagnoses”, “Treatment”, “Management”, and others into search engines, including PubMed, Web of Science, EMBASE, Google Scholar, and Medline-ProQuest. The search and selection process for the references that are included in this article were independently performed by four authors (C.C.Y.W., H.H.L.W., I.P., and A.W.).
2. Epidemiology of CKDaP
The reported prevalence of CKDaP from major national and international registries is hugely variable [
20,
21,
22,
23,
24]. This may be explained by different levels of renal and dialysis care provision, different perceptions of patients and relatives, and variable levels of awareness of CKDaP [
25]. It is likely that the current studies underestimate the true prevalence of CKDaP [
20,
21,
22,
23]. For example, the dialysis outcomes and practice patterns study (DOPPS) highlighted that 17% of patients who were receiving hemodialysis (HD) did not report this to their attending clinician [
21]. Only 1% of clinicians correctly estimated the prevalence range of severe pruritus in dialysis centers where the true prevalence was between 21% and 50% [
21].
The epidemiological data on CKDaP within specific treatment groups for patients receiving HD, peritoneal dialysis (PD), kidney transplantation, and non-dialysis treatment are discussed herein. Across the study phases in the DOPPS, the proportion of patients with moderate to severe pruritic symptoms did not significantly differ between the study phases, hovering above 40% [
20,
21,
22]. The reported prevalence of CKDaP in populations that were receiving PD is much more variable, ranging between 10% and 70% [
26]. PD is highlighted as an independent risk factor for CKDaP, following adjustments for confounding variables, such as age, body mass index, and dialysis modality [
27]. Nevertheless, there were no adjustments for biochemical parameters, with this being a potentially confounding factor influencing the findings [
28]. Evidence is relatively scarce on the prevalence of pruritic symptoms in post-transplant patients, though this is believed to lie between the range of 12% and 32%, according to a previous literature review of eight studies [
10]. The data from the chronic kidney disease outcomes and practice patterns study (CKDOPPS) and the chronic kidney disease-renal epidemiology and information network (CKD-REIN) studies explored the prevalence of CKDaP amongst non-dialysis CKD patients [
23]. The proportion of patients with moderate to extreme levels of pruritus was 24% in CKDOPPS and CKD-REIN, with up to 13% of patients experiencing severe to extreme pruritic symptoms [
23]. The patients living with kidney failure have reported a 19% greater prevalence of moderate to extreme itch compared with those with stage 3 CKD [
23].
There has been an emergence of epidemiological data describing the non-modifiable and modifiable risk factors of CKDaP. The earlier phases of the DOPPS data have reported higher calcium–phosphate product concentration correlating with pruritic severity amongst HD patients, though the relationship that exists between calcium–phosphate levels and pruritic symptoms remains debatable without further convincing data [
21]. The evidence in regard to the impact of age, sex, ethnicity, and country of residence on CKDaP remains controversial and, therefore, further study is needed [
14,
20,
21,
22,
23,
24,
28]. Multiple co-morbidities have demonstrated strong associations with increased severity of CKDaP. These included cardiovascular, lung, neurological, and chronic infectious diseases [
20,
28]. The relationship between diabetes mellitus and CKDaP has previously been relatively unclear, where higher and lower prevalence of CKDaP has been associated with diabetic patients, though recent studies have found that the primary cause of most cases of pruritus is prolonged poor diabetes control with altered glucose and insulin levels, subsequently causing skin dryness and neuropathy [
29,
30,
31]. Large population studies also did not indicate clear associations between laboratory parameters and CKDaP prevalence [
29,
30]. The data remain conflicting as to how anemia parameters, serum albumin, and inflammatory markers, such as C-reactive protein, white blood cells, and cytokines, correlate with pruritus severity in CKD [
20,
21,
28,
32,
33,
34,
35].
The negative impact of CKDaP on clinical outcomes has been concluded from major registry data. The evidence has remained consistent on the negative impact of CKDaP for HRQOL outcomes. In the DOPPS, patients receiving HD who were experiencing pruritic symptoms were more likely to report feeling washed out, having poor sleep, and depressive symptoms compared to patients without pruritic symptoms [
22]. The DOPPS also generated data concluding convincing associations between CKDaP and increased all-cause, infection-, skin-, infection-, and cardiovascular-related hospitalizations and mortality [
22]. The German epidemiological hemodialysis itch study (GEHIS) is another national registry study that explored the outcomes in patients with CKDaP. It highlighted the increased mortality amongst patients with CKDaP, particularly those who had skin lesions, elevated serum C-reactive protein, weaker physical conditioning, and depressive symptoms [
36]. Another conclusion of interest from the GEHIS is that patients who were receiving HD with pruritic symptoms were more likely to report pain and anxiety compared to those without pruritus [
24,
37]. The GEHIS also noted that more than 50% of patients who were receiving HD with pruritic symptoms complained to their clinicians regarding poor sleep, though there is no statistical correlation between the grade of pruritus severity and sleep quality [
24].
Given the holistic effects of CKDaP upon an individual’s physical, psychological, and functional well-being, healthcare professionals should invest greater attention on the psychosocial implications of CKDaP for both patients and their caregivers [
38]. In the DOPPS, CKDaP was found to be significantly associated with lower rates of employment [
22]. In countries where there may not be government initiatives to provide financial support/reimbursements for patients living with chronic diseases, such as CKD, financial difficulties with unemployment may add another source of burden for patients and their caregivers, contributing to increased depression and poorer adherence to care [
39,
40]. Whilst there is a relative lack of epidemiological data specifically evaluating the psychosocial implications of CKDaP at present, social deprivation has been established as an independent risk factor of negative overall outcomes in CKD [
41]. We anticipate more research assessing the psychosocial implications of CKDaP going forward.
3. Pathophysiology of CKDaP
Despite acknowledging the existence of CKDaP for many years, there remains no consensus regarding the etiology and pathophysiology of this phenomenon. In general terms, chronic pruritus is defined in cases where itching symptoms persist for over six weeks and is generally classified into the following five different categories: dermatological, systemic, neurological, somatoform, and mixed origin [
12,
13,
14,
16,
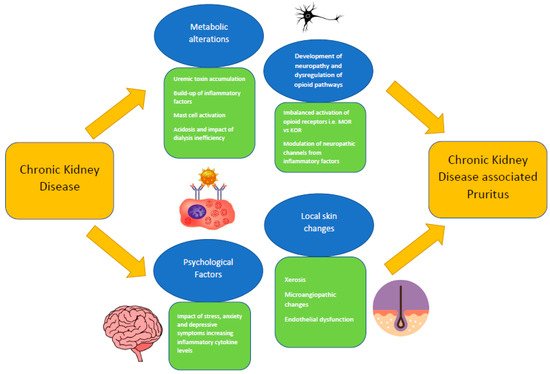
42]. CKDaP is thought to be a systemic condition that does not derive from a dermatological cause, as it occurs as the result of complex metabolic dysfunction. There are multiple pathophysiological mechanisms that are regarded to be major factors contributing towards the manifestation of CKDaP, and these mechanisms typically have overlapping characteristics (
Figure 1). Such mechanisms could be categorized into the following factors: local skin changes, metabolic alterations, development of neuropathy and dysregulation of opioid pathways, and psychological factors [
12,
13,
14,
16].
Figure 1. Overview of the pathophysiological processes of CKDaP.
Mucocutaneous changes are commonly observed in patients with CKD, and xerosis is frequently detected [
43,
44]. Xerosis of the skin in CKD is characterized by the atrophy of the secretory glands and a thickened basement membrane zone, resulting in an elevated pH alongside reduced hydration of the stratum corneum [
45,
46]. In most cases, the skin barrier function remains intact with CKD-related xerosis [
47,
48]. Pruritus is likely to improve with rehydration and continuous moisturization, which indicates the validity of this pathophysiological relationship [
39]. Previous studies assessing skin biopsy histological changes in CKD have noted microangiopathic changes and endothelial dysfunction [
49,
50,
51]. Microangiopathy is described as a spectrum of structural remodeling of the small vessels that leads to hypoxia, increased oxidative stress, increased apoptotic activity, a reduction in viable endothelial progenitor cells, and disturbed angiogenesis [
52]. Microangiopathic changes and endothelial dysfunction can cause capillary rarefication and fibrosis of the skin [
52].
Uremic toxin accumulation is highlighted in numerous studies to be a major cause of CKDaP, as this affects the homeostasis of various biochemical parameters ranging from calcium, phosphate, calcitriol, parathyroid hormone (PTH), magnesium, iron, bile acids, zinc, nitric oxide, and vitamin A [
20,
30,
53,
54,
55]. Hyperphosphatemia, hypocalcemia, and decreased calcitriol in CKD causes secondary hyperparathyroidism and, subsequently, CKD-MBD [
20,
30,
53,
54,
55]. The findings from previous studies suggest that CKD-MBD may impact upon the onset and severity of pruritic symptoms [
56]. This could be explained by the increase in calcium–phosphate product in CKD-MBD, leading to greater dermal calcium deposition, which in turn would be favored by elevated PTH levels [
57]. Observations that parathyroidectomy may cure CKDaP are supportive of this pathophysiological association [
58]. In addition to the uremic toxin build-up, a state of systemic micro-inflammation secondary to CKD is also thought to significantly contribute towards pruritic symptoms. Inflammatory markers, such as C-reactive protein and ferritin, and cytokines, such as interleukins: (IL-) 6, 31, and T-helper 1 cells, are documented to be elevated amongst HD patients and are recognized as factors contributing to the inflammatory process leading to CKDaP [
35,
59,
60,
61,
62]. Mast cells, which generate and release histamine, tryptase, eosinophils, protease, and IL-2, have been found to be at an increased level in patients with CKDaP [
12,
15,
30,
63]. These pruritogens are instrumental in the processes of skin proliferation and degranulation, triggering an itch response [
12,
15]. Other factors, such as the severity of acidosis and the effects of dialysis membrane permeability in dialysis-dependent patients, have been hypothesized, but will require further validation [
64,
65,
66,
67].
For patients with kidney failure who are receiving dialysis, there is an increased prevalence of peripheral sensorimotor neuropathy and dysautonomia [
68,
69]. This has been investigated as a potential explanation for the occurrence of CKDaP. It was observed that pruritus can be triggered by opioid agonists that are found on the brain, peripheral nerves, keratinocytes, melanocytes, hair follicles, and immune cells (granulocytes, monocytes/macrophages, and lymphocytes) [
12,
13,
14,
16,
70]. The three major opioid receptor types, with their corresponding ligands, are μ-opioid receptor (MOR) with endorphins, к-opioid receptor (KOR) with dynorphins, and δ-opioid receptor (DOR) with enkephalins [
71]. The KOR system is understood as an itch suppressor and the MOR system as an itch stimulator [
71]. The imbalanced activation between the MOR and KOR systems is hypothesized as a mechanism of CKDaP [
71]. Other receptors, such as morphine, endothelin-1, chloroquine, IL-13, and IL-31, have been proposed as key mediators of CKDaP [
72,
73]. The extent to which the inflammatory factors are involved in the modulation of the opioid system is also considered, given that the opioid receptors and the nerve terminals on the sensory nerves are upregulated during inflammation [
74,
75,
76,
77,
78,
79]. It has been suggested that the activation of the KOR system induces an anti-inflammatory response through the downregulation of cytokine, chemokine, and chemokine receptor expression [
80,
81]. These mechanisms are not well understood so far, therefore, there is a need for further experimental study.
The pathophysiological impact of psychological symptoms—notably stress, anxiety, and depression—have been considered to contribute towards CKDaP [
82]. Associations between inflammation, depression, and itching in dialysis patients have been described, with elevated inflammatory cytokine levels (C-reactive protein, IL-1, IL-6, IL-10, and tumor necrosis factor-α) co-existing in patients with depressive symptoms [
83,
84].
5. Prevention and Treatment of CKDaP
There are numerous pharmacological and non-pharmacological treatment options for CKDaP, but high-quality evidence that could be used to recommend one treatment approach over another is lacking and guidelines are sparse or non-existent, leading to a situation where, in effect, most nephrologists and departments have their own favored approach. An objective interpretation of the data from most studies is complicated by the variable methods of the outcomes assessment that is employed, and a lack of direct comparison between the different treatments. Another issue that has hampered research is that topical emollients, which are a mainstay of CKDaP treatment, are inexpensive and are, therefore, unattractive to the industry when it comes to funding large-scale clinical trials.
Given the burden of symptoms that are caused by CKDaP, and the fact that the symptoms can be debilitating, it is important to prioritize the prevention of CKDaP. The patients should be educated on good general skin care measures, such as avoiding soap-based cleansers, scratching (ideally keeping the fingernails short and clean), and triggers such as heat and stress. Additionally, a topical emollient should be considered for all patients with CKD, due to its ability to prevent the exacerbation of xerosis, which is highly prevalent in CKD and is a major contributor towards CKDaP [
89,
99]. Emollients with higher water content have been generally recommended. A previous study demonstrated a strong efficacy from the daily application of an aqueous gel containing 80% water content, with significant reductions in VAS over two weeks [
100]. Another small, randomized trial assessing the outcomes over one week found a reduction in pruritic symptoms with an emollient regime containing oil/water emulsion and glycerol/paraffin compared to oil/water emulsion alone amongst patients with CKD [
101]. Topical ointments containing omega 3- and omega 6-fatty acid and oiled bath preparations have demonstrated potential in the treatment of pruritic symptoms in CKD in recent studies, however, this requires more extensive validation [
102,
103,
104]. The specific evidence in regard to topical corticosteroid administration for CKDaP does not appear to be convincing at present, but topical corticosteroids are known to be effective for generalized pruritus (whether presenting with skin lesions or not), hence, they are commonly prescribed by clinicians who are caring for patients complaining of pruritic symptoms [
105].
Urea clearance and the effective management of CKD-associated complications are important aspects of treatment in order to reduce the severity of CKDaP symptoms. For dialysis-dependent patients, optimizing the uremic toxin removal through a trial of increased dialysis dosing should be the first step of CKDaP management [
67,
99]. Previous data remain controversial in regard to the optimal dialysis membrane and Kt/V targets to improve the pruritic symptoms for patients receiving HD [
67,
106]. Kt/V (‘K’ represents the dialyzer clearance of urea; ‘t’ represents the duration of the dialysis session; ‘V’ represents the volume of the distribution of urea, which is approximately equal to patient’s total body water) is a non-dimensional number and scaling parameter that been used as a measure of dialysis efficacy in a session of peritoneal dialysis (PD) or HD [
107]. Increasing ‘K’ by improving the vascular access in order to achieve a better blood flow rate during dialysis and increasing ‘t’ through extending dialysis for a longer period in each dialysis session are considered to be ways of improving the overall Kt/V [
108]. However, the urea accumulation and removal are affected by a broad spectrum of factors, which can confound the accuracy of Kt/V [
109]. There remains ongoing work to find solutions to improve the utilization of Kt/V in dialysis practices.
Most, if not all, of the reported studies relating to dialysis-dependent patients with CKDaP have small sample sizes, and therefore larger, prospective, multi-center studies are needed in order to determine the optimal dialysis regime for this group. Kidney transplantation, if it is appropriate for the individual patient with kidney failure, appears to be a superior treatment option to dialysis due to its potential to restore kidney function and to significantly improve uremic clearance and the CKD-associated complications [
70,
110]. Small prospective cohort studies have illustrated consistency in the resolution of pruritic symptoms and skin changes for previous CKDaP patients who underwent kidney transplantation [
111,
112,
113]. The post-transplant skin biopsies found the previously noticeable uremic skin alterations to have been resolved [
110,
111]. If the non-dialysis conservative management of CKD is deemed to be the most suitable option, then there should be a primary focus on reducing the risks and the impacts of CKD-associated complications, such as CKD-MBD [
99]. Whilst dietary phosphate restriction and calcium-based/aluminum-based/phosphate-binding medications are commonly prescribed first-line treatment options, parathyroidectomy may be utilized in severe cases when the patients are refractory to dietary and medical therapy [
114,
115].
This entry is adapted from the peer-reviewed paper 10.3390/allergies2030009