Plant lesion mutation refers to the phenomenon whereby plants spontaneously form necrotic spots of different sizes on the leaves, leaf sheaths, stems, and even the grains without external abiotic stress or biotic stress [
1,
2]. Related studies have shown that the generation process of plant lesion traits is very similar to the symptoms of the plant hypersensitivity response (HR) in vivo. The HR in plants is an innate immune mechanism for fighting against pathogen infection in vivo [
3]. Plants further acquire systemic acquired immunity (SAR) by activating the HR process, thereby enhancing plant resistance. The HR is also a type of programmed cell death (PCD), and lesion mimic mutant plants generally exhibit the phenomenon of cell death, which results in premature senescence, consequently impacting the agronomic traits of some crops [
4,
5]. In addition, some studies have confirmed that most plant lesion mutants can enhance the resistance to plant pathogens.
2. Discovery, Excavation, and Classification of Rice Lesion Mimic Mutants
Since the first lesion mimic mutant
sekiguchi lesion (
SL) in rice was reported in the mid-1960s [
8], researchers have identified a series of other similar mutants. However, relatively few lesion mimic mutants have been produced under natural mutation, and most have been obtained by artificial mutation. The first cloned lesion mimic gene in rice was
Spotted Leaf 7 (
SPL7), and the mutant
spl7, which had small red-brown lesions distributed on the entire leaf surface, was induced by γ-ray irradiation [
9]. The
SPL7 gene encodes a heat shock transcription factor, negatively regulating cell apoptosis under high-temperature stress after mutation. Subsequently, homologous genes of
SPL7 were found in maize, Arabidopsis, tomato, and other plants, which could produce lesion mimic traits in the corresponding plants [
10]. In the lesion mimic mutants, gene mutation breaks the metabolic balance in the plant, leading to the appearance of abnormal phenotype and other damages, such as the lessening of the chlorophyll content, decrease of photosynthesis and plant height, and the final reduction of crop yield [
11]. For example, the leaves of rice
lesion mimic and senescence mutant 1 (
lms1) began to appear as russet brown spots at the late tillering stage, and the disease spots spread to the whole leaves and even stems with the plant growth.
Most of the lesions appear on the leaves or leaf sheaths in rice and are often brown, red-brown, dark brown, and dark yellow, among which brown lesions are the most common [
14]. Lesions throughout the whole growth period, from seedling stage to mature seed stage leaves, always have obvious lesions. At the initial stage of vegetative growth, lesions appear between one month and two months after sowing. Some lesion mimic mutants continue to display the characteristics of lesions at the whole growth stage, and some lesions do not show any longer. Initiation lesions of reproductive growth occur only at the late stage of growth, and some occur after heading until seed maturation [
15]. Therefore, lesion mimic mutants can be divided into whole life lesion mimics (WLLMs) (such as
lrd35,
lrd40), vegetative initiation lesion mimics (VILMs) (such as
lrd41,
lrd44), and reproductive initiation lesion mimics (RILMs) (such as
lrd27,
lrd28,
lrd39) according to the occurrence period of the lesion mimic mutations. Another classification is to classify lesion mimic mutants into initiation class and propagation class according to their phenotypes [
16]. In addition, lesion mimic mutants can also be divided into environmentally sensitive mutants and environmentally insensitive mutants according to the environmental sensitivity of the lesion mimic traits [
14].
3. Mechanism of Rice Lesion Occurrence
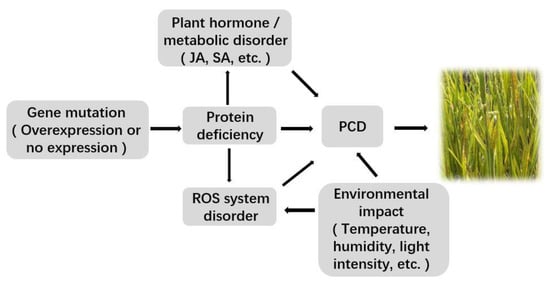
The mechanism of lesion mimic occurrence in rice is extremely complex and is mainly regulated by disease resistance mechanisms, death regulation, and related metabolic enzymes (Figure 1). The changes in various signaling pathways in rice and the stress of some external environmental factors are also very important factors in forming a lesion.
Figure 1. Mechanism of rice lesion mimic occurrence. (The mechanism of plant lesion formation is varied and complex and mainly regulated by genes such as disease resistance, death regulation, and basic metabolic enzymes; plant defense signaling molecules and external environmental factors also play important roles in forming lesions. All the arrows indicated positive regulatory relations).
3.1. Related Gene Mutation or Expression Change
In rice, if the disease-resistant or stress-resistant genes are mutated, it may cause disorder of the signaling pathways, resulting in PCD and further causing lesion mimic phenotype. For example, the rice
NLS1 gene encodes a coiled-coil nucleotide-binding leucine-rich repeat-like (CC-NB-LRR-like) R protein. After the mutation of
NLS1, excessive H
2O
2 and salicylic acid (SA) accumulated in the mutant, and resistance-related genes were also upregulated, which led to the spontaneous formation of lesion mimic spots on the leaf sheath [
17]. In addition, the phenotype of the dominant
Spl18 mutant was related to the insertion of the T-DNA activation label, which enhanced the transcriptional level of rice acyltransferase
OsAT1, and caused lesion mimicry of
Spl18. Meanwhile, the expression levels of resistance-related genes were upregulated with the severity of the lesion mimicry in
Spl18 [
18]. In addition to rice, such phenomena have also been observed in other plants, such as with the
SSI gene in Arabidopsis. The expression of defense genes in mutant Arabidopsis plants increased significantly, resulting in the lesion mimic phenomenon [
19].
3.2. Accumulation of ROS
Reactive oxygen species are single-electron-reduction products of oxygen in vivo and mainly include hydroxyl radicals, hydrogen peroxide, and superoxide anion radicals. When the concentration of ROS in plants is lower than a certain value, the plant defense system is activated, and the ROS participates in the signal transduction of cells in plants. However, when the concentration of ROS exceeds a certain level, it will have toxic effects on cells and can even lead to cell death [
20]. It has been found that plant lesion mutants typically produce a large amount of ROS during the formation of necrotic lesions. These high concentrations of ROS can destroy the normal structure of the cells and can also be used as signaling molecules to induce HRs, leading to cell death [
20]. For example, the rice lesion mimic gene
SPL5 encodes a hypothetical transcription splicing factor 3b subunit 3 (SF3b3), which makes the mutant accumulate excessive superoxide anion and hydrogen peroxide, leading to the appearance of the lesion mimic phenotype in the mutant [
21]. The same is true for the
nitric oxide excess1 (
noe1) mutant in rice. The
NOE1 gene encodes a catalase OsCATC, which is responsible for eliminating hydrogen peroxide. So, the mutation of
NOE1 led to the production of excess hydrogen peroxide in leaves and the excessive accumulation of nitric oxide in the mutant because of the activation of nitrate reductase, which ultimately resulted in the exhibition of a lesion mimic phenotype in
noe1 [
22].
3.3. Effects of Plant Hormones
In the process of plant lesion formation, some plant hormones, including ethylene (ET), jasmonic acid (JA), and salicylic acid (SA), play important roles in regulating different defense responses [
23,
24]. The
OsEDR1 gene in rice positively regulates the synthesis of ET, and the knockout (KO) of
OsEDR1 inhibits the ACC synthase genes, which encode the rate-limiting enzymes of ET biosynthesis resulting in the decreased production of ET. Meanwhile, the SA and JA contents in the
OsEDR1-KO plants were increased, indicating that when the function of the
OsEDR1 gene was missing, the plant hormone level would be unbalanced, thus inducing necrotic lesions on the leaves at the booting stage [
25]. Salicylic acid is one of the key hormones affecting plant resistance. In a study of the
OsSSI2 gene in rice, it was found that the transposon insertion mutants of this gene and RNA interference (RNAi) plants all showed lesion mimic traits, and the resistance of plants to
Magnaporthe grisea was enhanced. Further studies showed that the endogenous SA content in transgenic plants was significantly increased [
26]. In addition, when pathogens invade plants, the content of JA rapidly accumulates in plants and activates defense responses. As in lesion mimic mutants
oshpl3 and
spl29, the JA content was significantly enhanced, and the expression levels of defense-related genes were upregulated [
27,
28].
3.4. Disorder of Plant Metabolic Pathways
In addition to being influenced by plant hormones, plant growth and metabolism are also regulated by many enzymes and proteins. If these enzymes and proteins are inactivated in the process of metabolism, they will cause metabolic disorders during plant growth, resulting in the emergence of lesion mimic phenotypes. The
FGL gene of rice encodes a protochlorophyllide oxidoreductase B (OsPORB), which catalyzes the photoreduction of protochlorophyllide to chlorophyllide under high light conditions. After the mutation, the chlorophyll metabolism of
fgl mutant was disordered, and the leaf of
fgl in color was rapidly changed from green to yellow and then formed lesions during leaf elongation in field-grown conditions [
29]. The
SPL28 gene encodes a grid-associated receptor protein complex, namely the central subunit μ1 (AP1M1), which participates in the vesicle transport process of the Golgi body. The mutation of
SPL28 disordered the normal material transport of the Golgi body, and the red-brown spots on leaves of
spl28 mutant began to emerge at the early tillering stage and reach the maximum number at the heading stage [
30].
3.5. Uncontrolled PCD
Programmed cell death is a common death mode in the development of organisms and is determined by genes. In the plant growth process, once the normal PCD in the body is disordered, it may lead to abnormal growth and development [
31]. The appearance of the lesion mimic phenotype in plants is also one of the manifestations of spontaneous cell death. In rice, the
SPL11 gene encodes an E3 ubiquitin ligase, negatively regulating PCD. The mutation of this gene led to a change in E3 ubiquitin ligase activity, resulting in the generation of lesion mimic spots [
32]. In addition, in rice, the
Oryza sativa accelerated cell death and resistance 1 (
OsACDR1) gene and
G-box factor 14-3-3 homologs (
GF14e) gene are also related to PCD reaction.
OsACDR1, also named
OsEDR1, encodes a putative Raf-like mitogen-activated protein kinase kinase kinase (MAPKKK), which can inhibit OsMPKK10.2 activity through physical interaction. The mutation of
OsACDR1 released the inhibition of OsMPKK10.2, so the activation of the pathogen-inducible OsMPKK10.2-OsMPK6 cascade was amplified [
33]. Meanwhile, the mutation promoted plant cell death, resulting in the lesion mimic phenotype, and the defense-related genes were upregulated [
25,
33].
GF14e, encoding a 14-3-3 protein, is induced during effector-triggered immunity (ETI) associated with pathogens.
GF14e has a negative regulatory effect on cell death and the defense response of rice. Therefore, its RNAi plants exhibited the lesion mimic character as well as increased resistance to bacterial blight and other pathogens [
34].
3.6. Influence of the External Environment
When a plant is subjected to abiotic environmental stresses, such as light, temperature, or humidity, it will elicit a series of immune stress responses to environmental stress. This process is often accompanied by the accumulation of ROS and the initiation of PCD process, resulting in the appearance of a lesion mimic phenotype. The rice
lesions stimulating disease resistance 1 (
OsLSD1) gene can be induced under light conditions and inhibited under dark conditions. Moreover,
OsLSD1 plays a negative regulatory role in PCD, and so, the
lsd1 mutant can produce lesion mimic trait under light [
35]. In addition, the mutant
lesion mimic and premature senescence 1 (
lmps1) are also affected by light. When part of the leaf was shaded, the lesion mimic phenotype did not appear on the mutant leaves, but following the reintroduction of light, the lesion mimic character appeared again on the leaves [
36]. A study on six
lrd lesion mimic mutants in rice found that high temperature could inhibit the lesion mimic trait of mutants
lrd31,
lrd35, and
lrd40, while low temperature could significantly promote the occurrence of lesion mimic traits (except for the
lrd31), and at a normal temperature of 28 °C, all
lrd mutants produced lesion mimic traits on the leaves [
37]. Therefore, environmental factors such as light intensity and temperature also affect plant lesion mimic production.
4. Identification, Cloning, and Functional Analysis of Rice Lesion Mimic Genes
With the development of molecular biology, many studies have been conducted on rice lesion mimic mutant materials. More than 70 lesion mimic mutants have been identified in rice, and some have been successfully cloned (
Table 1). According to the genetic characteristics of the identified rice lesion mimic mutants, most of them are controlled by single recessive genes [
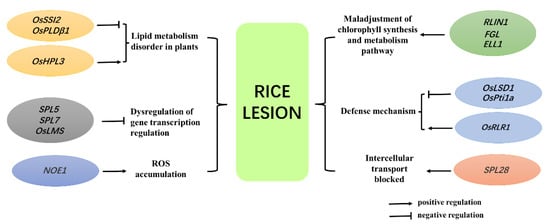
1]. Function analysis of the cloned lesion mimic genes indicated that these genes are mainly involved in lipid metabolism, gene transcription regulation, chlorophyll metabolism, and plant defense responses (
Figure 2).
Figure 2. Part of the regulation pathway of lesion mimic genes in rice. (The regulatory pathways involved in rice lesion mimic genes mainly include lipid metabolism and chlorophyll synthesis metabolism in plants, transcription pathways of plant cell death, defense pathways of plants, intercellular transport pathways, and ROS signal transduction pathways).
Table 1. Partially cloned 35 rice lesion mimic genes.
|
Mutant Gene
|
Mutant Type
|
Accession Number
|
Protein Function Analysis
|
Reference
|
|
SPL7
|
RILM
|
Os05g05304
|
Heat shock transcription factor
|
[9]
|
|
SPL11
|
RILM
|
Os12g05700
|
E3 ubiquitin ligase
|
[32]
|
|
LSD1
|
VILM
|
Os08g01595
|
C2C2 zinc finger protein
|
[35]
|
|
OsNPR1(NH1)
|
VILM
|
Os01g01943
|
Transcriptional coactivators
|
[38]
|
|
SPL18(OsAT1)
|
WLLM
|
Os10g01956
|
Acyltransferase
|
[18]
|
|
OsPti1a
|
VILM
|
Os05g01358
|
Rice protein kinase
|
[39]
|
|
XB15
|
VILM
|
Os03g60650
|
Protein phosphatase
|
[40]
|
|
OsACDR1
(OsEDR1,SPL3)
|
RILM
|
Os03g01601
|
Mitogen-activated protein kinase
|
[33]
|
|
OsSSI2
|
RILM
|
Os01g09199
|
Fatty acid dehydrogenase
|
[26]
|
|
OsPLDβ1
|
VILM
|
Os10g38060
|
Phospholipase
|
[41]
|
|
SPL28
|
RILM
|
Os01g07036
|
Subunits of grid-related receptor protein complexes
|
[30]
|
|
OsSL(ELL1)
|
RILM
|
Os12g02680
|
Cytochrome P450 monooxygenase
|
[42,43]
|
|
RLIN1
|
WLLM
|
Os04g06108
|
Porphyrin III oxidase
|
[44]
|
|
GF14e
|
WLLM
|
Os02g05803
|
14-3-3 protein
|
[34]
|
|
NOE1
|
RILM
|
Os03g03910
|
Catalase
|
[35]
|
|
NLS1
|
RILM
|
Os11g14380
|
CC-NB-LRR type R protein
|
[17]
|
|
SPL5
|
RILM
|
Os07g02037
|
Splicing factor 3b subunit
|
[45]
|
|
OsHPL3
|
VILM
|
Os02g01102
|
Hydroperoxide lyase
|
[28,46]
|
|
OsLMS
|
WLLM
|
Os02g06390
|
RNA binding protein
|
[47]
|
|
CslF6
|
VILM
|
Os08g01605
|
Cellulose-like synthase F
|
[48]
|
|
RLS1
|
VILM
|
Os02g10900
|
NB-ARM domain protein
|
[49]
|
|
FGL
|
VILM
|
Os10g35370
|
OsPORB protein, involved in cytochrome synthesis
|
[29]
|
|
SPL29
|
WLLM
|
Os08g02069
|
Acetylglucosamine pyrophosphatase
|
[27]
|
|
LMR
|
RILM
|
Os06g01300
|
Adenosine triphosphatase
|
[50]
|
|
EBR1
|
VILM
|
Os05g19970
|
E3 ubiquitin ligase in RING domain
|
[51]
|
|
OsPLS1
|
RILM
|
Os06g45120
|
Vacuolar proton ATPase subunit
|
[52]
|
|
SPL32
|
VILM
|
Os07g06584
|
Ferroxin-dependent glutamate synthase
|
[53]
|
|
SPL33
|
WLLM
|
Os01g01166
|
eEF1A-like protein
|
[54]
|
|
OsCUL3a
|
VILM
|
Os02g07460
|
Cullin protein
|
[55]
|
|
LML1
|
WLLM
|
Os04g06599
|
Eukaryotic Release Factor 1 Protein
|
[56]
|
|
SDS
|
VILM
|
Os01g57480
|
SD-1 receptor-like kinase
|
[57]
|
|
SPL35
|
WLLM
|
Os03g02050
|
CUE domain protein
|
[58]
|
|
SPL40
|
WLLM
|
Os05g03120
|
Ribosome structural components
|
[59]
|
|
LMM24
|
VILM
|
Os03g24930
|
receptor-like cytoplasmic kinase
|
[60]
|
|
OsRLR1
|
RILM
|
Os10g07978
|
CC-NB-LRR protein
|
[61]
|
The lesion mimic mutants can be divided into whole life lesion mimics (WLLMs), vegetative initiation lesion mimics (VILMs), and reproductive initiation lesion mimics (RILMs) according to the occurrence period of the lesion mimic mutations.
5. Disease Resistance of Rice Lesion Mimic Mutants
Currently, according to the reported results, most rice lesion mimic mutants show enhanced resistance to pathogens. For example, compared with the wild type, the
lsd1 mutant significantly improved the resistance to bacterial and fungal pathogens [
35]. The rice lesion mimic mutant
spl28 significantly increased resistance to bacterial blight and rice blast due to the accumulation of large amounts of callose in the plants [
30]. In addition,
spl10,
cdr3, and other mutants showed enhanced resistance to rice blast;
spl21,
lmes1,
hm83, and other mutants exhibited enhanced resistance to bacterial blight, and the mutant
lmm1 was resistant to both sheath blight and blast [
1]. These studies showed that the lesion mimic mutant gene might activate the immune stress response in plants due to the loss of function, thereby enhancing the resistance to pathogens. Therefore, it also shows that the lesion mimic mutant of rice is a good material for studying plant PCD procedures and disease resistance and defense mechanisms.