DNA methylation is an epigenetic mark that living beings have used in different environments. The MTases family catalyzes DNA methylation. This process is conserved from archaea to eukaryotes, from fertilization to every stage of development, and from the early stages of cancer to metastasis. The family of DNMTs has been classified into DNMT1, DNMT2, and DNMT3. Each DNMT has been duplicated or deleted, having consequences on DNMT structure and cellular function, resulting in a conserved evolutionary reaction of DNA methylation. DNMTs are conserved in the five kingdoms of life: bacteria, protists, fungi, plants, and animals.
- DNA methylation
- MTases
- RNAs
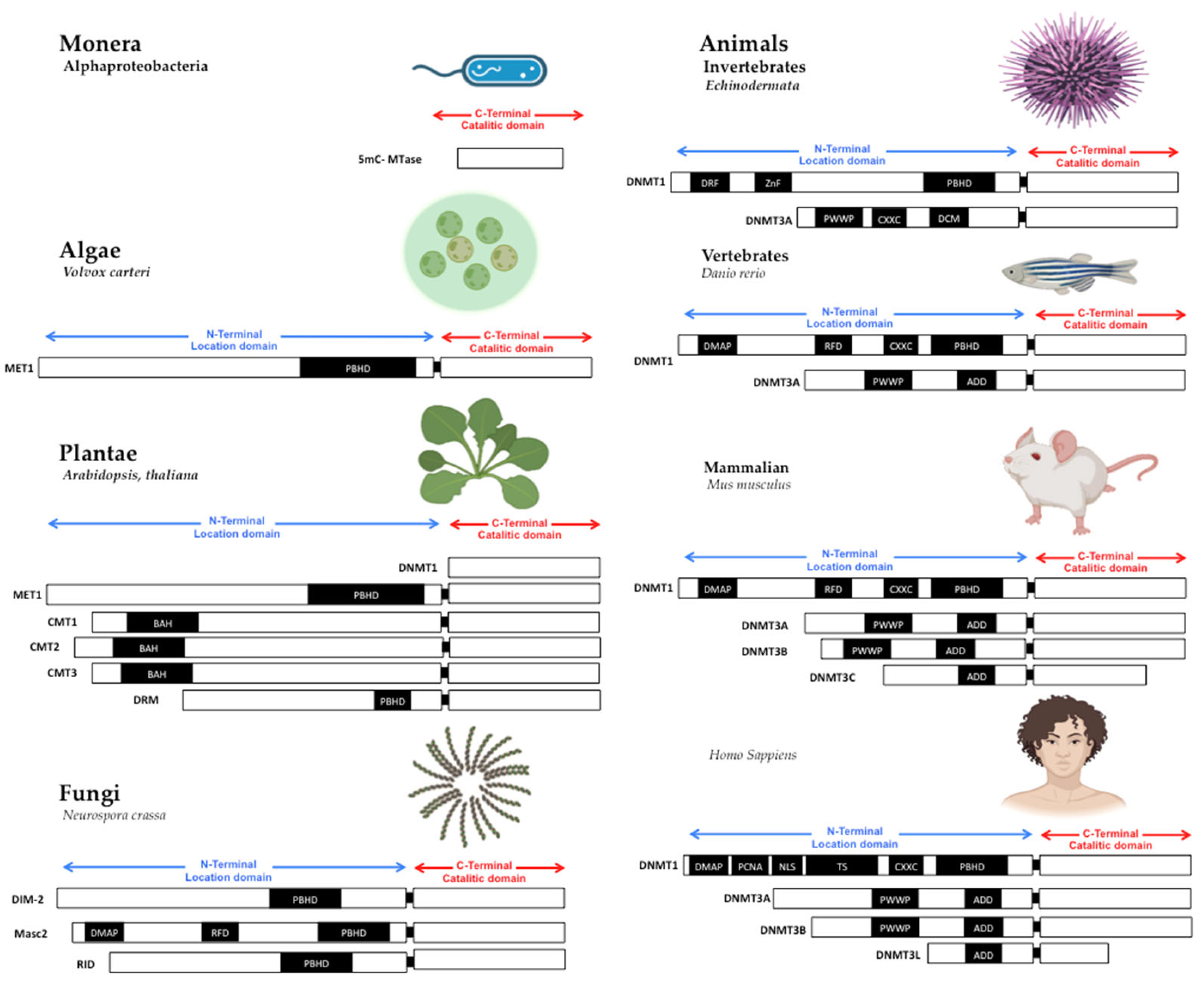
1. The Structure of DNA Methyltransferases
2. DNA Methyltransferases Are Regulated by Chemical Compounds and ncRNAs
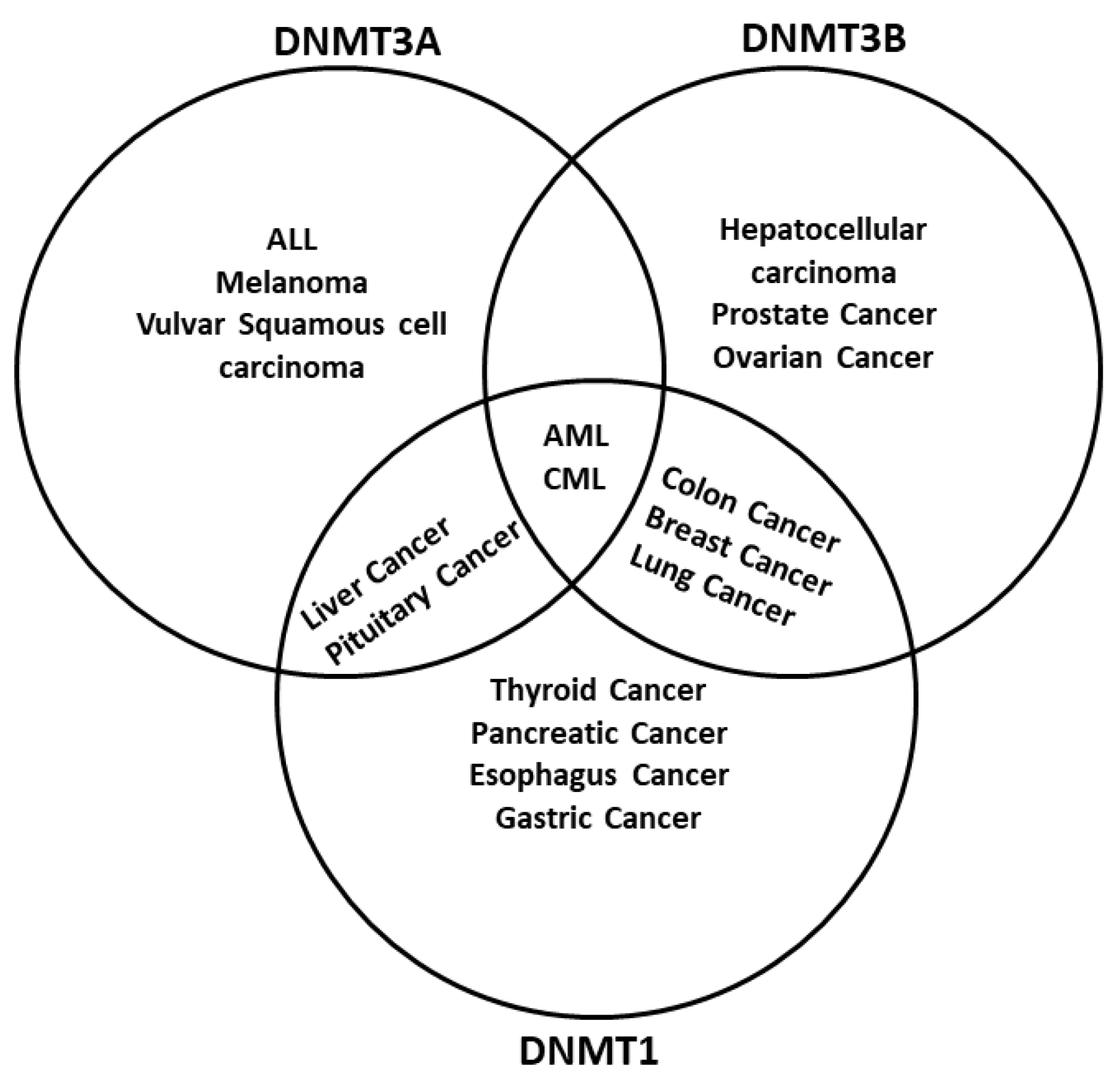
| ncRNAs | DNMT Deregulated in Cancer |
Type of Cancer | Type of Deregulation | Reference |
|---|---|---|---|---|
| DBCCR1-003 | DNMT1 | Bladder | Down | [35] |
| linc-POU3F3 | DNMT1, 3A, and 3B | ESCC | Up | [36] |
| miR-148a | DNMT1 | Gastric | Down | [37] |
| miR-29a | DNMT1 | Liver | Down | [38] |
| miR-152 | DNMT1 | Glioma | Down | [39] |
| miR-185 | DNMT1 | Glioma | Down | [40] |
| miR-145 | DNMT3A | Ovarian | Down | [41] |
| miR-101 | DNMT3A | Glioma | Down | [42] |
| miR-29 | DNMT3B | Burkitt | Down | [43] |
| miR-29b | DNMT3B | Lymphoma, pancreatic, head and neck cell line cancer |
Down | [44][45] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms23168994
References
- Ponger, L.; Li, W.-H. Evolutionary Diversification of DNA Methyltransferases in Eukaryotic Genomes. Mol. Biol. Evol. 2005, 22, 1119–1128.
- Campos, C.; Valente, L.M.P.; Fernandes, J.M.O. Molecular Evolution of Zebrafish Dnmt3 Genes and Thermal Plasticity of Their Expression during Embryonic Development. Gene 2012, 500, 93–100.
- Mosquera-Rendón, J.; Cárdenas-Brito, S.; Pineda, J.D.; Corredor, M.; Benítez-Páez, A. Evolutionary and Sequence-Based Relationships in Bacterial AdoMet-Dependent Non-Coding RNA Methyltransferases. BMC Res. Notes 2014, 7, 440.
- Zhenilo, S.V.; Sokolov, A.S.; Prokhortchouk, E.B. Epigenetics of Ancient DNA. Acta Nat. 2016, 30, 72–76.
- Jurkowski, T.P.; Jeltsch, A. On the Evolutionary Origin of Eukaryotic DNA Methyltransferases and Dnmt2. PLoS ONE 2011, 6, e28104.
- Bheemanaik, S.; Reddy, Y.V.R.; Rao, D.N. Structure, Function and Mechanism of Exocyclic DNA Methyltransferases. Biochem. J. 2006, 399, 177–190.
- Chédin, F. The DNMT3 Family of Mammalian De Novo DNA Methyltransferases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 101, pp. 255–285.
- Jurkowska, R.Z.; Jurkowski, T.P.; Jeltsch, A. Structure and Function of Mammalian DNA Methyltransferases. Chembiochem 2011, 12, 206–222.
- Lee, J.-H.; Voo, K.S.; Skalnik, D.G. Identification and Characterization of the DNA Binding Domain of CpG-Binding Protein. J. Biol. Chem. 2001, 276, 44669–44676.
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenetics Chromatin 2017, 10, 23.
- Xu, T.-H.; Liu, M.; Zhou, X.E.; Liang, G.; Zhao, G.; Xu, H.E.; Melcher, K.; Jones, P.A. Structure of Nucleosome-Bound DNA Methyltransferases DNMT3A and DNMT3B. Nature 2020, 586, 151–155.
- Taverna, S.D.; Li, H.; Ruthenburg, A.J.; Allis, C.D.; Patel, D.J. How Chromatin-Binding Modules Interpret Histone Modifications: Lessons from Professional Pocket Pickers. Nat. Struct. Mol. Biol. 2007, 14, 1025–1040.
- Bestor, T.H. Cytosine Methylation Mediates Sexual Conflict. Trends Genet. 2003, 19, 185–190.
- Goyal, R. Accuracy of DNA Methylation Pattern Preservation by the Dnmt1 Methyltransferase. Nucleic Acids Res. 2006, 34, 1182–1188.
- Fatemi, M.; Hermann, A.; Pradhan, S.; Jeltsch, A. The Activity of the Murine DNA Methyltransferase Dnmt1 Is Controlled by Interaction of the Catalytic Domain with the N-Terminal Part of the Enzyme Leading to an Allosteric Activation of the Enzyme after Binding to Methylated DNA. J. Mol. Biol. 2001, 309, 1189–1199.
- Li, E.; Zhang, Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133.
- Feinberg, A.P.; Vogelstein, B. Hypomethylation Distinguishes Genes of Some Human Cancers from Their Normal Counterparts. Nature 1983, 301, 89–92.
- Feinberg, A.P.; Vogelstein, B. A Technique for Radiolabeling DNA Restriction Endonuclease Fragments to High Specific Activity. Anal. Biochem. 1983, 132, 6–13.
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.; et al. DNMT3A Mutations in Acute Myeloid Leukemia. N. Engl. J. Med. 2010, 363, 2424–2433.
- Kanai, Y.; Ushijima, S.; Nakanishi, Y.; Sakamoto, M.; Hirohashi, S. Mutation of the DNA Methyltransferase (DNMT) 1 Gene in Human Colorectal Cancers. Cancer Lett. 2003, 192, 75–82.
- Amatori, S.; Bagaloni, I.; Donati, B.; Fanelli, M. DNA Demethylating Antineoplastic Strategies: A Comparative Point of View. Genes Cancer 2010, 1, 197–209.
- Huang, D.; Cui, L.; Ahmed, S.; Zainab, F.; Wu, Q.; Wang, X.; Yuan, Z. An Overview of Epigenetic Agents and Natural Nutrition Products Targeting DNA Methyltransferase, Histone Deacetylases and MicroRNAs. Food Chem. Toxicol. 2018, 123, 574–594.
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA Methylation Profiles in Cancer Diagnosis and Therapeutics. Clin. Exp. Med. 2018, 18, 1–14.
- Gnyszka, A.; Jastrzebski, Z.; Flis, S. DNA Methyltransferase Inhibitors and Their Emerging Role in Epigenetic Therapy of Cancer. Anticancer Res. 2013, 33, 2989–2996.
- Daher-Reyes, G.S.; Merchan, B.M.; Yee, K.W.L. Guadecitabine (SGI-110): An Investigational Drug for the Treatment of Myelodysplastic Syndrome and Acute Myeloid Leukemia. Expert Opin. Investig. Drugs 2019, 28, 835–849.
- Datta, J.; Ghoshal, K.; Denny, W.A.; Gamage, S.A.; Brooke, D.G.; Phiasivongsa, P.; Redkar, S.; Jacob, S.T. A New Class of Quinoline-Based DNA Hypomethylating Agents Reactivates Tumor Suppressor Genes by Blocking DNA Methyltransferase 1 Activity and Inducing Its Degradation. Cancer Res. 2009, 69, 4277–4285.
- Rilova, E.; Erdmann, A.; Gros, C.; Masson, V.; Aussagues, Y.; Poughon-Cassabois, V.; Rajavelu, A.; Jeltsch, A.; Menon, Y.; Novosad, N.; et al. Design, Synthesis and Biological Evaluation of 4-Amino-N-(4-Aminophenyl)Benzamide Analogues of Quinoline-Based SGI-1027 as Inhibitors of DNA Methylation. Chemmedchem 2014, 9, 590–601.
- Zwergel, C.; Schnekenburger, M.; Sarno, F.; Battistelli, C.; Manara, M.C.; Stazi, G.; Mazzone, R.; Fioravanti, R.; Gros, C.; Ausseil, F.; et al. Identification of a Novel Quinoline-Based DNA Demethylating Compound Highly Potent in Cancer Cells. Clin. Epigenetics 2019, 11, 68.
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA Methyltransferase Inhibitors Combination Therapy for the Treatment of Solid Tumor: Mechanism and Clinical Application. Clin. Epigenetics 2021, 13, 166.
- Ahuja, N.; Sharma, A.R.; Baylin, S.B. Epigenetic Therapeutics: A New Weapon in the War Against Cancer. Annu. Rev. Med. 2016, 67, 73–89.
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long Non-Coding RNAs: Mechanism of Action and Functional Utility. Non-Coding RNA Res. 2016, 1, 43–50.
- Klisovic, R.B.; Stock, W.; Cataland, S.; Klisovic, M.I.; Liu, S.; Blum, W.; Green, M.; Odenike, O.; Godley, L.; Burgt, J.V.; et al. A Phase I Biological Study of MG98, an Oligodeoxynucleotide Antisense to DNA Methyltransferase 1, in Patients with High-Risk Myelodysplasia and Acute Myeloid Leukemia. Clin. Cancer Res. 2008, 14, 2444–2449.
- Winquist, E.; Knox, J.; Ayoub, J.-P.; Wood, L.; Wainman, N.; Reid, G.K.; Pearce, L.; Shah, A.; Eisenhauer, E. Phase II Trial of DNA Methyltransferase 1 Inhibition with the Antisense Oligonucleotide MG98 in Patients with Metastatic Renal Carcinoma: A National Cancer Institute of Canada Clinical Trials Group Investigational New Drug Study. Investig. New Drugs 2006, 24, 159–167.
- Plummer, R.; Vidal, L.; Griffin, M.; Lesley, M.; de Bono, J.; Coulthard, S.; Sludden, J.; Siu, L.L.; Chen, E.X.; Oza, A.M.; et al. Phase I Study of MG98, an Oligonucleotide Antisense Inhibitor of Human DNA Methyltransferase 1, Given as a 7-Day Infusion in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2009, 15, 3177–3183.
- Qi, D.; Li, J.; Que, B.; Su, J.; Li, M.; Zhang, C.; Yang, M.; Zhou, G.; Ji, W. Long Non-Coding RNA DBCCR1-003 Regulate the Expression of DBCCR1 via DNMT1 in Bladder Cancer. Cancer Cell Int. 2016, 16, 81.
- Li, W.; Zheng, J.; Deng, J.; You, Y.; Wu, H.; Li, N.; Lu, J.; Zhou, Y. Increased Levels of the Long Intergenic Non-Protein Coding RNA POU3F3 Promote DNA Methylation in Esophageal Squamous Cell Carcinoma Cells. Gastroenterology 2014, 146, 1714–1726.
- Yan, J.; Guo, X.; Xia, J.; Shan, T.; Gu, C.; Liang, Z.; Zhao, W.; Jin, S. MiR-148a Regulates MEG3 in Gastric Cancer by Targeting DNA Methyltransferase 1. Med. Oncol. 2014, 31, 879.
- Braconi, C.; Kogure, T.; Valeri, N.; Huang, N.; Nuovo, G.; Costinean, S.; Negrini, M.; Miotto, E.; Croce, C.M.; Patel, T. MicroRNA-29 Can Regulate Expression of the Long Non-Coding RNA Gene MEG3 in Hepatocellular Cancer. Oncogene 2011, 30, 4750–4756.
- Zhang, P.; Sun, H.; Yang, B.; Luo, W.; Liu, Z.; Wang, J.; Zuo, Y. MiR-152 Regulated Glioma Cell Proliferation and Apoptosis via Runx2 Mediated by DNMT1. Biomed. Pharmacother. 2017, 92, 690–695.
- Zhang, Z.; Tang, H.; Wang, Z.; Zhang, B.; Liu, W.; Lu, H.; Xiao, L.; Liu, X.; Wang, R.; Li, X.; et al. MiR-185 Targets the DNA Methyltransferases 1 and Regulates Global DNA Methylation in Human Glioma. Mol. Cancer 2011, 10, 1–16.
- Zhang, S.; Pei, M.; Li, Z.; Li, H.; Liu, Y.; Li, J. Double-negative Feedback Interaction between DNA Methyltransferase 3A and MicroRNA-145 in the Warburg Effect of Ovarian Cancer Cells. Cancer Sci. 2018, 109, 2734–2745.
- Liu, X.; Lei, Q.; Yu, Z.; Xu, G.; Tang, H.; Wang, W.; Wang, Z.; Li, G.; Wu, M. MiR-101 Reverses the Hypomethylation of the LMO3 Promoter in Glioma Cells. Oncotarget 2015, 6, 7930.
- Mazzoccoli, L.; Robaina, M.C.; Apa, A.G.; Bonamino, M.; Pinto, L.W.; Queiroga, E.; Bacchi, C.E.; Klumb, C.E. MiR-29 Silencing Modulates the Expression of Target Genes Related to Proliferation, Apoptosis and Methylation in Burkitt Lymphoma Cells. J. Cancer Res. Clin. Oncol. 2018, 144, 483–497.
- Wang, L.; Huang, J.; Wu, C.; Huang, L.; Cui, J.; Xing, Z.; Zhao, C. Downregulation of MiR-29b Targets DNMT3b to Suppress Cellular Apoptosis and Enhace Proliferation in Pancreatic Cancer. Mol. Med. Rep. 2018, 17, 2113–2120.
- Chen, L.-H.; Hsu, W.-L.; Tseng, Y.-J.; Liu, D.-W.; Weng, C.-F. Involvement of DNMT 3B Promotes Epithelial-Mesenchymal Transition and Gene Expression Profile of Invasive Head and Neck Squamous Cell Carcinomas Cell Lines. BMC Cancer 2016, 16, 431.
- Jones, R.; Wijesinghe, S.; Wilson, C.; Halsall, J.; Liloglou, T.; Kanhere, A. A Long Intergenic Non-Coding RNA Regulates Nuclear Localization of DNA Methyl Transferase-1. Iscience 2021, 24, 102273.
- Somasundaram, S.; Forrest, M.E.; Moinova, H.; Cohen, A.; Varadan, V.; LaFramboise, T.; Markowitz, S.; Khalil, A.M. The DNMT1-Associated LincRNA DACOR1 Reprograms Genome-Wide DNA Methylation in Colon Cancer. Clin. Epigenetics 2018, 10, 127.
- Guo, X.; Chen, Z.; Zhao, L.; Cheng, D.; Song, W.; Zhang, X. Long Non-Coding RNA-HAGLR Suppressed Tumor Growth of Lung Adenocarcinoma through Epigenetically Silencing E2F1. Exp. Cell Res. 2019, 382, 111461.
