Toxoplasma gondii is an obligate intracellular parasite that causes toxoplasmosis, with approximately one third of the population around the world seropositive. The consumption of contaminated food is the main source of infection. These include meat products with T. gondii tissue cysts, and dairy products with tachyzoites.
- toxoplasmosis
- Toxoplasma gondii
- control
- food
- detection
1. Introduction
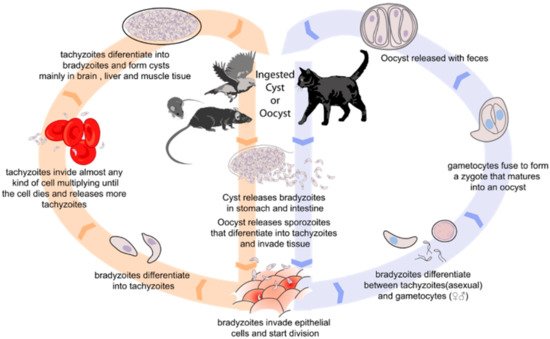
2. Methods for T. gondii Detection in Food Products
|
Detection Method |
Specific Method 1 |
Type of Food Product |
Detection Range (Sensitivity) 2 |
References |
|---|---|---|---|---|
|
Animal model bioassay |
Cat |
Milk |
25% |
|
|
Meat |
100% |
[31] |
||
|
Mouse |
Milk |
100% |
[29] |
|
|
Meat |
100% (10 tachyzoites) |
[24] |
||
|
Fresh products |
13% |
[32] |
||
|
Bivalve mollusks |
2.5% |
[33] |
||
|
Water |
100% |
[34] |
||
|
Cell culture |
Meat |
100% (10,000 tachyzoites) |
[24] |
|
|
Milk |
- |
[30] |
||
|
Microscopic method |
Meat |
- |
[31] |
|
|
Molecular methods |
PCR |
Meat |
47.1% |
[35] |
|
Fresh products |
95–100% |
|||
|
Water |
100% |
[36] |
||
|
Milk |
100% |
|||
|
Cheese |
100% |
[29] |
||
|
qPCR |
Meat |
92.3% (limit 0.01 pg) |
||
|
Fresh products |
100% (1 oocyst) |
|||
|
Bivalve mollusks |
100% |
[44] |
||
|
Water |
100% |
[44] |
||
|
LAMP |
Lymph nodes |
85.7% |
[45] |
|
|
Mussels |
5 oocyst/g |
[46] |
||
|
Fresh products |
25 oocyst/50 g |
[47] |
||
|
Water |
100% (1 fg) |
|||
|
Serological methods |
IHA |
Meat Juice |
100% (10,000 oocysts) |
[50] |
|
IFAT |
Meat |
97% |
[51] |
|
|
Meat Juice |
96.9% (10,000 oocysts) |
[50] |
||
|
MAT |
Meat |
86.6% |
[51] |
|
|
Milk |
- |
[52] |
||
|
ELISA |
Milk |
- |
[30] |
|
|
Meat |
91% |
[51] |
||
|
Meat Juice |
100% (10,000 oocysts) |
[50] |
||
|
BBMA |
Meat |
98.5% |
[53] |
1 PCR: Polymerase chain reaction; qPCR: real-time PCR; LAMP: Loop-mediated isothermal amplification; IHA: indirect hemagglutination antibody; IFAT: indirect fluorescent antibody test; MAT: modified agglutination test; ELISA: Enzyme-Linked Immunosorbent Assay; BBMA: bead-based multiplex assay. 2 The column shows the percentage of samples that were positively detected by the method and the quantity of parasites per quantity of food product that was detected if this data is known. The value (-) means that this data is not known.
3. Prevalence of Toxoplasma gondii in Food Products
3.1. Meat and Meat Products
|
Animal |
Sample Analyzed |
Detection Method 1 |
Number of Samples Tested |
Number of Positive Samples (%) |
Location |
Reference |
|---|---|---|---|---|---|---|
|
Sheep |
Serum |
ELISA |
150 |
26 (17.3%) |
Iran |
[89] |
|
Serum |
ELISA |
550 |
59 (10.8%) |
Iran |
[90] |
|
|
Serum |
ELISA |
1039 |
179 (17.2%) |
Latvia |
[91] |
|
|
Serum |
MAT |
100 |
42 (42%) |
Lebanon |
[92] |
|
|
Serum |
ELISA |
64 |
30 (47%) |
Slovakia |
[93] |
|
|
Serum |
DAT |
252 |
148 (58.2%) |
Ethiopia |
[94] |
|
|
Liver |
PCR |
150 |
26 (17.3%) |
Iran |
[89] |
|
|
Liver |
PCR |
90 |
13 (14.4%) |
Iran |
[95] |
|
|
Heart |
PCR |
150 |
48 (32%) |
Iran |
[89] |
|
|
Brain and heart |
MAT |
136 |
10 (7.4%) |
India |
[96] |
|
|
Meat juice |
ELISA |
227 |
126 (28.6%) |
Italy |
[97] |
|
|
Meat juice |
MAT |
166 |
11 (6.6%) |
China |
[98] |
|
|
Meat |
PCR |
150 |
33 (22%) |
Iran |
[89] |
|
|
Meat |
PCR |
438 |
43 (9.8) |
China |
[99] |
|
|
Meat |
PCR |
150 |
50 (33.3) |
Tunisia |
[100] |
|
|
Meat |
ELISA |
109 |
38 (34.9%) |
Malaysia |
[101] |
|
|
Meat |
PCR |
79 |
34 (43%) |
Australia |
[102] |
|
|
Meat |
PCR |
177 |
3 (1.7%) |
India |
[103] |
|
|
Goat |
Serum |
ELISA |
150 |
16 (10.7%) |
Iran |
[89] |
|
Serum |
ELISA |
185 |
37 (20%) |
Iran |
[90] |
|
|
Serum |
ELISA |
445 |
189 (42.5%) |
India |
[104] |
|
|
Serum |
MAT |
80 |
27 (34%) |
Lebanon |
[93] |
|
|
Serum |
ELISA |
39 |
8 (21%) |
Slovakia |
[93] |
|
|
Serum |
LAT |
116 |
64 (55.2%) |
Ethiopia |
[94] |
|
|
Liver |
PCR |
150 |
24 (16%) |
Iran |
[89] |
|
|
Liver |
PCR |
90 |
8 (8.8%) |
India |
[95] |
|
|
Heart |
PCR |
150 |
36 (24%) |
Iran |
[89] |
|
|
Brain and heart |
MAT |
57 |
4 (7%) |
India |
[96] |
|
|
Meat juice |
ELISA |
51 |
14 (27.5%) |
Italy |
[97] |
|
|
Meat |
PCR |
150 |
26 (17.3%) |
Iran |
[89] |
|
|
Meat |
PCR |
254 |
27 (10.7) |
China |
[99] |
|
|
Meat |
PCR |
120 |
39 (32.5) |
Tunisia |
[100] |
|
|
Meat |
ELISA |
75 |
41 (54.7%) |
Malaysia |
[101] |
|
|
Meat |
PCR |
223 |
3 (1.3%) |
India |
[104] |
|
|
Cattle |
Serum |
ELISA |
57 |
13 (22.8%) |
Italy |
[105] |
|
Serum |
DAT |
2411 |
313 (13%) |
Poland |
[106] |
|
|
Serum |
ELISA |
400 |
52 (13%) |
Iran |
[107] |
|
|
Serum |
IFAT |
500 |
2.3 (40.6%) |
Brazil |
[108] |
|
|
Meat |
PCR |
150 |
29 (19.3) |
Tunisia |
[100] |
|
|
Meat |
ELISA |
392 |
98 (25%) |
Malaysia |
[101] |
|
|
Meat |
PCR |
48 |
5 (10.4%) |
Brazil |
[108] |
|
|
Pig |
Serum |
ELISA |
653 |
4 (0.6%) |
Finland |
[109] |
|
Serum |
ELISA |
447 |
73 (16.3%) |
Denmark |
[110] |
|
|
Serum |
DAT |
3111 |
370 (11.9%) |
Poland |
[106] |
|
|
Serum |
IFAT |
94 |
44 (46.8%) |
Romania |
[111] |
|
|
Serum |
ELISA |
420 |
56 (23.3%) |
Cuba |
[112] |
|
|
Serum |
ELISA |
370 |
14 (3.8%) |
Italy |
[113] |
|
|
Serum |
ELISA and IFAT |
127 |
56 (44.1%) |
Italy |
[114] |
|
|
Serum |
MAT |
375 |
8 (2.1%) |
Italy |
[115] |
|
|
Serum |
ELISA |
414 |
214 (51.7%) |
Italy |
[116] |
|
|
Serum |
MAT |
182 |
31 (17%) |
Serbia |
[117] |
|
|
Serum |
MAT and IFAT |
356 |
25 (7%) and 48 (13.5%), respectively |
Brazil |
[118] |
|
|
Serum |
MAT and IFAT |
400 |
26 (6.5%) |
Brazil |
[119] |
|
|
Serum |
IFAT |
60 |
44 (77%) |
Brazil |
[120] |
|
|
Serum |
IHA |
784 |
156 (19.9%) |
China |
[121] |
|
|
Tongue |
PCR |
60 |
20 (33.3%) |
Brazil |
[120] |
|
|
Tongue and muscle |
PCR |
810 |
54 (6.7%) |
India |
[122] |
|
|
Brain |
PCR |
339 |
34 (10%) |
China |
[123] |
|
|
Brain |
PCR |
107 |
51 (47.7%) |
Italy |
[116] |
|
|
Heart |
PCR |
94 |
25 (26.6%) |
Romania |
[111] |
|
|
Heart |
qPCR |
103 |
12 (11.6%) |
Italy |
[124] |
|
|
Diaphragm |
PCR |
45 |
15 (33.3%) |
Serbia |
[117] |
|
|
Diaphragm |
PCR |
1223 |
107 (8.7%) |
China |
[125] |
|
|
Diaphragm |
PCR |
60 |
24 (40%) |
Brazil |
[120] |
|
|
Diaphragm |
qPCR |
103 |
2 (1.9%) |
Italy |
[126] |
|
|
Tissue of seropositive animals |
Mouse bioassay |
26 |
18 (69.2%) |
Brazil |
[119] |
|
|
Muscle |
PCR |
60 |
23 (38.3%) |
Brazil |
[120] |
|
|
Meat juice |
ELISA |
212 |
33 (15.6%) |
Denmark |
[110] |
|
|
Meat |
qPCR |
118 |
46 (39%) |
Brazil |
[126] |
|
|
Meat |
PCR |
498 |
165 (33.1%) |
Italy |
[64] |
|
|
Meat |
PCR |
49 |
3 (6.1%) |
Brazil |
[108] |
|
|
Raw meat products |
PCR |
3223 |
175 (5.4%) |
Poland |
[127] |
|
|
Chicken |
Serum |
IFAT |
200 |
72 (36%) |
Brazil |
[128] |
|
Serum |
ELISA |
522 |
34 (6.5%) |
India |
[129] |
|
|
Serum |
LACA |
267 |
29 (10.9%) |
Japan |
[85] |
|
|
Brain |
Mouse Bioassay |
14 |
2 (14.3%) |
Brazil |
[128] |
|
|
Heart juice |
MAT |
1185 |
230 (19.4%) |
USA |
[130] |
|
|
Muscle and heart |
PCR |
522 |
12 (2.3%) |
India |
[129] |
|
|
Meat |
PCR |
257 |
21 (8.2%) |
China |
[131] |
|
|
Ducks |
Meat |
PCR |
115 |
9 (7.8%) |
China |
[131] |
|
Geese |
Meat |
PCR |
42 |
2 (4.8%) |
China |
[131] |
|
Rabbit |
Brain and heart |
PCR |
470 |
13 (2.8%) |
China |
[132] |
|
Kibbeh |
Meat |
PCR |
44 |
1 (2.3%) |
Brazil |
[108] |
|
Water Buffalo |
Serum |
MAT and ELISA |
197 |
16 (8.1%) and 13 (6.6%), respectively |
Romania |
[133] |
|
Ostriches (farmed) |
Serum |
LAT |
409 |
149 (36%) |
Czech Republic |
[134] |
|
Common quails (farmed) |
Serum |
MAT |
620 |
59 (9.5%) |
China |
[135] |
|
Donkey (farmed) |
Meat |
PCR |
618 |
57 (9.2%) |
China |
[136] |
|
Tolai hares (farmed) |
Serum |
PCR |
358 |
29 (8.1%) |
China |
[137] |
|
Brain |
PCR |
358 |
23 (6.4%) |
China |
[137] |
|
|
Feral swine |
Serum |
ELISA |
376 |
34 (9%) |
USA |
[138] |
|
Wild boar (farmed) |
Serum |
LAT |
882 |
88 (10%) |
China |
[139] |
|
Wild boar |
Serum |
ELISA |
331 |
164 (49%) |
Italy |
[140] |
|
Serum |
ELISA |
181 |
17 (9%) |
Finland |
[141] |
|
|
Serum |
IFAT |
26 |
20 (76.9%) |
Brazil |
[142] |
|
|
Serum |
ELISA |
306 |
61 (20%) |
Germany |
[143] |
|
|
Tissue |
Mouse bioassay |
22 |
1 (4.5%) |
Brazil |
[142] |
|
|
Brain |
qPCR |
141 |
44 (31.2%) |
Italy |
[144] |
|
|
Brain |
PCR |
263 |
58 (22%) |
Italy |
[145] |
|
|
Heart |
qPCR |
166 |
47 (28.3%) |
Italy |
[144] |
|
|
Heart |
PCR |
310 |
70 (22.6%) |
Italy |
[145] |
|
|
Muscle |
qPCR |
165 |
40 (24.2%) |
Italy |
[144] |
|
|
Muscle |
PCR |
311 |
74 (23.8%) |
Italy |
[145] |
|
|
Meat juice |
ELISA |
97 |
42 (43.3%) |
Italy |
[146] |
|
|
Meat |
qPCR |
306 |
37 (12%) |
Germany |
[143] |
|
|
Venison |
Serum |
MAT |
914 |
329 (36%) |
USA |
[147] |
|
Heart |
Mouse bioassay |
36 |
11 (30.6%) |
USA |
[147] |
|
|
Roe deer |
Serum |
LAT |
356 |
141 (39.6%) |
Spain |
[148] |
|
Serum |
ELISA |
323 |
130 (40.2%) |
Italy |
[149] |
|
|
Serum |
ELISA |
184 |
20 (11%) |
Germany |
[143] |
|
|
Meat |
qPCR |
184 |
11 (6%) |
Germany |
[143] |
|
|
Fallow deer |
Serum |
LAT |
372 |
138 (37.1%) |
Spain |
[150] |
|
Serum |
ELISA |
167 |
17 (10%) |
Slovakia |
[93] |
|
|
Meat |
qPCR |
80 |
2 (2%) |
Germany |
[143] |
|
|
Red deer |
Serum |
LAT |
553 |
92 (16.6%) |
Spain |
[148] |
|
Serum |
ELISA |
96 |
19 (19.8%) |
Italy |
[140] |
|
|
Serum |
ELISA |
65 |
4 (6%) |
Germany |
[143] |
|
|
Meat |
qPCR |
65 |
2 (2%) |
Germany |
[143] |
|
|
Southern chamois |
Serum |
LAT |
186 |
26 (14%) |
Spain |
[148] |
|
Mouflon |
Serum |
LAT |
209 |
24 (11.5%) |
Spain |
[148] |
|
Serum |
ELISA |
50 |
12 (24%) |
Italy |
[140] |
|
|
Iberian wild goat |
Serum |
LAT |
346 |
27 (7.8%) |
Spain |
[148] |
|
Chamois |
Serum |
ELISA |
104 |
4 (3.8%) |
Italy |
[140] |
|
Barbary sheep |
Serum |
LAT |
18 |
1 (5.6%) |
Spain |
[148] |
|
Moose |
Serum |
DAT |
463 |
111 (23.9%) |
Estonia |
[149] |
|
Wild ducks |
Brain |
qPCR |
280 |
7 (2.5%) |
Czech Republic |
[150] |
|
Heart |
qPCR |
280 |
11 (3.9%) |
Czech Republic |
[150] |
|
|
Muscle |
qPCR |
280 |
4 (1.4%) |
Czech Republic |
[150] |
|
|
Common pheasants |
Brain |
qPCR |
350 |
8 (2.3%) |
Czech Republic |
[150] |
|
Heart |
qPCR |
350 |
4 (1.1%) |
Czech Republic |
[150] |
|
|
Muscle |
qPCR |
350 |
3 (0.9%) |
Czech Republic |
[150] |
1 ELISA: Enzyme-Linked Immunosorbent Assay; MAT: modified agglutination test; DAT: direct agglutination test; PCR: Polymerase chain reaction; LAT: latex agglutination test; IFAT: indirect fluorescent antibody test; qPCR: real-time PCR.
3.2. Milk and Dairy Products
|
Animal |
Sample Analyzed |
Detection Method 1 |
Number of Samples Tested |
Number of Positive Samples (%) |
Location |
Reference |
|---|---|---|---|---|---|---|
|
Donkey |
Milk |
ELISA |
418 |
41 (9.2%) |
China |
[167] |
|
Goat |
Milk |
ELISA |
30 |
19 (63.3%) |
Italy |
[172] |
|
Milk |
PCR |
60 |
39 (65%) |
Poland |
[157] |
|
|
Milk |
ELISA and qPCR |
30 |
27 (90%) and 1 (3.3%), respectively |
Egypt |
[173] |
|
|
Bulk tank milk |
ELISA |
100 |
59 (59%) |
Italy |
[172] |
|
|
Sheep |
Milk |
PCR |
58 |
1 (1.7%) |
Mongolia |
[168] |
|
Milk |
ELISA and qPCR |
30 |
18 (60%) and 1 (3.3%), respectively |
Egypt |
[173] |
|
|
Camel |
Milk |
PCR |
9 |
8 (88.9%) |
Mongolia |
[168] |
|
Milk |
ELISA and qPCR |
30 |
1 (3.33%) and 0 (0%), respectively |
Egypt |
[173] |
|
|
Cattle |
Bulk tank milk |
ELISA |
149 |
8 (5.4%) |
Iran |
[174] |
1 ELISA: Enzyme-Linked Immunosorbent Assay; PCR: Polymerase chain reaction; qPCR: real-time PCR.
3.3. Fresh Products and Vegetables
|
Product Analyzed |
Detection Method 1 |
Number of Samples Tested |
Number of Positive Samples (%) |
Location |
Reference |
|---|---|---|---|---|---|
|
Mixed-salad packages |
qPCR |
648 packages |
5 (0.8%) |
Italy |
[183] |
|
PCR |
90 packages |
8 (8.9%) |
Czech Republic |
[184] |
|
|
Leafy greens |
qPCR |
152 |
45 (29.6%) |
Morocco |
[185] |
|
Carrot |
qPCR |
30 |
3 (10%) |
Morocco |
[186] |
|
qPCR |
46 |
9 (19.5%) |
Poland |
[177] |
|
|
PCR |
93 |
7 (7.5%) |
Czech Republic |
[184] |
|
|
Chicory |
PCR |
40 |
2 (5%) |
Brazil |
[187] |
|
Red cabbage |
qPCR |
8 |
1 (1.2%) |
China |
[42] |
|
Coriander |
qPCR |
29 |
8 (27.6%) |
Morocco |
[186] |
|
Cucumber |
PCR |
109 |
13 (11.9%) |
Czech Republic |
[184] |
|
Lettuce |
qPCR |
28 |
3 (10.7%) |
Morocco |
[186] |
|
qPCR |
50 |
9 (18%) |
Poland |
[177] |
|
|
qPCR |
71 |
5 (7%) |
China |
[42] |
|
|
PCR |
168 |
5 (3%) |
Brazil |
[187] |
|
|
Spinach |
qPCR |
50 |
2 (4%) |
China |
[42] |
|
Parsley |
qPCR |
29 |
13 (44.8%) |
Morocco |
[186] |
|
PCR |
5 |
1 (20%) |
Brazil |
[187] |
|
|
Pak Choi |
qPCR |
34 |
1 (2.9%) |
China |
[42] |
|
Radish |
qPCR |
16 |
1 (6.3%) |
Morocco |
[186] |
|
qPCR |
60 |
3 (5%) |
Poland |
[42] |
|
|
Rape |
qPCR |
22 |
1 (4.5%) |
China |
[42] |
|
Rocket |
PCR |
7 |
1 (14.3%) |
Brazil |
[187] |
1 PCR: Polymerase chain reaction; qPCR: real-time PCR.
3.4. Marine Products
|
Animal |
Sample Analyzed |
Detection Method 1 |
Number of Samples Tested |
Number of Positive Samples (%) |
Location |
Reference |
|---|---|---|---|---|---|---|
|
Bivalve shellfish |
Tissue |
PCR |
2907 |
82 (2.8%) |
China |
[194] |
|
Green-lipped mussels |
Tissue |
PCR |
104 |
13 (16.4%) |
New Zealand |
[195] |
|
Mediterranean mussel |
Gills |
qPCR |
53 pools at 795 specimens |
21 (39.6%) |
Turkey |
[189] |
|
Clam |
Tissue |
qPCR |
61 pools at 1020 specimens |
4 (6.6%) |
Tunisia |
[190] |
|
Digestive gland |
PCR |
390 |
6 (1.5%) |
Canada |
[196] |
|
|
Haemolymph |
PCR |
390 |
2 (0.6%) |
Canada |
[196] |
|
|
Mediterranean scald fish |
Gills |
PCR |
1 pool at 6 specimens |
1 (100%) |
Italy |
[197] |
|
Pacific oyster |
Gills |
PCR |
6 pools at 109 specimens |
1 (16.67%) |
Italy |
[198] |
|
Oyster |
Mantle, gills, and rectum |
qPCR |
1440 |
447 (31%) |
USA |
[199] |
|
Bogue |
Gills |
PCR |
26 pools at 260 specimens |
4 (15.4%) |
Italy |
[197] |
|
Intestine |
PCR |
26 pools at 260 specimens |
3 (11.5%) |
Italy |
[197] |
|
|
Muscle |
PCR |
26 pools at 260 fish |
6 (23.1%) |
Italy |
[197] |
|
|
White seabream |
Muscle |
PCR |
3 pools of 18 specimens |
1 (33.3%) |
Italy |
[197] |
|
European anchovy |
Gills |
PCR |
35 pools at 350 specimens |
2 (5.7%) |
Italy |
[197] |
|
Intestine |
PCR |
35 pools at 350 specimens |
1 (2.9%) |
Italy |
[197] |
|
|
European hake |
Gills |
PCR |
15 pools at 90 specimens |
1 (6.7%) |
Italy |
[197] |
|
Muscle |
PCR |
15 pools at 90 specimens |
1 (6.7%) |
Italy |
[197] |
|
|
Red mullet |
Intestine |
PCR |
11 pools at 110 specimens |
3 (27.3%) |
Italy |
[197] |
|
American prawn |
Muscle |
PCR |
618 |
4 |
China |
[197] |
|
Nippon shrimp |
Muscle |
PCR |
813 |
1 |
China |
[200] |
|
Axillary seabream |
Gills |
PCR |
8 pools at 80 specimens |
2 (25%) |
Italy |
[197] |
|
Intestine |
PCR |
8 pools at 80 specimens |
1 (12.5%) |
Italy |
[197] |
|
|
Muscle |
PCR |
8 pools at 80 specimens |
1 (12.5%) |
Italy |
[197] |
|
|
Common pandora |
Gills |
PCR |
3 pools at 18 specimens |
1 (33.3%) |
Italy |
[197] |
|
Intestine |
PCR |
3 pools at 18 specimens |
2 (66.7%) |
Italy |
[197] |
|
|
Muscle |
PCR |
3 pools at 18 specimens |
1 (33.3%) |
Italy |
[197] |
|
|
Thornback ray |
Muscle |
PCR |
1 fish |
1 (100%) |
Italy |
[198] |
|
Red scorpionfish |
Intestine |
PCR |
1 pool at 3 specimens |
1 (100%) |
Italy |
[197] |
|
Blotched picarel |
Muscle |
PCR |
4 pools at 24 specimens |
1 (25%) |
Italy |
[197] |
|
Atlantic horse mackerel |
Muscle |
PCR |
15 pools at 120 specimens |
4 (26.7%) |
Italy |
[197] |
1 PCR: Polymerase chain reaction; qPCR: real-time PCR.
4. Control and Food Safety
This entry is adapted from the peer-reviewed paper 10.3390/foods11162542
