2. Classification of Acute Pancreatitis
Performing specific diagnostic measures should be adjusted to the presumed cause and condition of the patient. Firstly, the patient has to meet two of the three criteria to be diagnosed with AP. Subsequently, local complications are assessed using diagnostic imaging methods, and systemic complications are evaluated based on the assessment of the efficiency of the respiratory, cardiovascular, and urinary systems. These complications determine the severity of the AP, which affects the management with the patient
[1][5].
AP is diagnosed on the basis of two of three criteria—typically belt-like abdominal pain, an elevated serum lipase level three times above the normal threshold, and radiological imaging signs of pancreatitis
[4][5][6]. The first two are present in the most of patients, whereas the latter occurs slightly less frequently. Due to that, in the vast majority of cases, diagnosis of AP can already be established on the basis of abdominal pain and an elevation of pancreatic enzymes
[6].
The revised Atlanta classification system from 2012
[5], defining the clinical diagnosis, CT manifestations, and the disease course of acute pancreatitis, distinguishes two morphologic subtypes of AP: interstitial oedematous pancreatitis and necrotizing pancreatitis.
The aforementioned classification, evaluating additional local or systemic complications as well as the presence and duration of organ failure, divides AP into three subtypes: mild AP, moderately severe AP, and severe AP
[3][5]. The mortality is different among the subtypes of AP. For instance, severe, necrotizing AP leads to a mortality rate reaching 25%, while mild, edematous AP includes a mortality of only 1%. Twenty to thirty percent of patients suffering from AP experience recurrent pancreatitis attacks, and of these, 10% develop CP
[7][8][9].
Pancreatitis can lead to local or systemic complications. Each of those has its own characteristics based on the patient’s symptoms. AP can be a mild, self-limiting disease that requires only supportive measures, but it also might turn into a severe disorder with life-threatening complications, such as insufficiency of the respiratory and cardiovascular systems or kidney failure
[2][5]. The characteristics of the aforementioned complications and the subtypes of acute pancreatitis are described in the table below (
Table 1).
Table 1. Classification of acute pancreatitis based on the 2012 Atlanta Classification of Acute Pancreatitis. Explanation of crucial terms.
The RAC and DBC are similar in determining the diagnosis and severity of AP. The RAC, in addition to the severity classification, clearly defines the definition of AP, highlights the onset of pain, and defines individual local complications, as well as interstitial and necrotizing pancreatitis
[1][3]. The RAC has three categories: mild, moderately severe, and severe. The DBC added a fourth category—critical, based on two major determinants of mortality: organ failure and (peri)pancreatic necrosis. Persistent organ failure with infected necrosis is associated with the highest risk of death. Therefore, those patients should be admitted to an intensive care unit and constantly monitored. Accordingly, the diagnosis and prediction of severe AP is crucial, as is the identification of the patients with a high risk of developing complications
[3].
3. Diagnosis of Acute Pancreatitis
According to the recommendations, the diagnosis of acute pancreatitis is based on blood tests to determine the level of serum lipase and amylase and imaging techniques: magnetic resonance cholangiopancreatography (MRCP), CT, and US
[2]. Measurements of serum and urinary enzymes are used to diagnose the AP, none of them allow the evaluation of the severity of the AP and the accurate prediction of the clinical course of the disease
[10][11][12].
Nowadays, due to technological development, radiologic imaging plays a more and more significant role in the management of the patient. The above-mentioned imaging techniques provide crucial information for the diagnosis and the course of the disease. Specifically, ultrasonography is recommended as a first and basic imaging test performed in patients with suspected AP in order to confirm or exclude the diagnosis as well as detect the possible cause of the disease, while MRI and CT are useful in diagnosing local complications and discovering the necrosis of the pancreas or in assessing the severity of the AP. The latter two have specific indications; they are performed to broaden the diagnosis or when ultrasound does not visualize the structures properly, making it impossible to make an unequivocal diagnosis
[13]. It is worth emphasizing that the diagnosis of AP is as important as the diagnosis of the etiology of AP, which in many cases is associated with an inadequate workup.
3.1. Laboratory Test and Indicator Enzymes
People suffering from the acute onset of a persistent, diffuse abdominal pain or acute epigastric pain should be diagnosed for acute pancreatitis. Therefore, it is important to know the diagnostic accuracy of serum lipase, serum amylase, urinary trypsinogen-2, and urinary amylase, either alone or in combination, for the diagnosis of AP
[14].
The accurate diagnosis of AP, the early assessment of the severity of AP, and the identification of the etiology are criteria that should be met by an ideal laboratory test in assessing the condition of a patient with AP. Currently, no biochemical test has been identified that fulfils the above-mentioned criteria and can be considered the “gold standard” for the diagnosis and evaluation of the severity of AP
[10].
Nonetheless, the relevant and currently commonly used laboratory tests in the diagnosis of AP are serum lipase and serum amylase
[10]. Based on multiple studies, lipase serum has been found to be a more reliable indicator of AP than serum amylase, whereas a reliable early diagnosis of AP is assured by urinary strip tests for trypsinogen-2 and trypsinogen activation peptide (TAP)
[15]. Other enzymes used in the diagnosis of AP, such as pancreatic isoamylase, immunoreactive trypsin, chymotrypsin, or elastase, are not better than lipase; furthermore, they are more inconvenient and expensive. Measurement of the levels of the aforementioned enzymes should be reserved for situations of uncertain diagnosis. Neither enzyme assay is associated with the severity of AP and cannot precisely predict the consecutive clinical course of the patient
[10][11][12]. According to Al-Bahrani and Ammori
[15], a biliary etiology is reliably predicted by early transient hypertransaminasemia, whereas a reliable predictor of alcoholic etiology is serum carbohydrate-deficient transferrin
[15]. Urinary enzymes are less significant in clinical practice than serum enzymes among the adults. However, urinary enzymes can be used in the case of AP in children
[16]. Nevertheless, it is worth knowing all the available diagnostic methods.
3.2. Laboratory Tests
3.2.1. Serum Lipase and Amylase
According to a clinical practice guideline, published in 2016 by Greenberg et al.
[2][17], concerning the management of acute pancreatitis, in all patients with suspicion of acute pancreatitis a level of serum lipase should be tested because of its slightly higher (79%) sensitivity in comparison with other serum and urine tests (72%). The diagnosis of acute pancreatitis is made when a serum lipase activity is at least three times greater than the upper limit of normal.
A serum amylase test is also performed in the diagnostics of AP, but it has a lower clinical value. The key blood biochemical parameter in the detection of acute pancreatitis is a serum lipase, which is characterized by an earlier and longer-lasting elevation than a serum amylase. Specifically, the lipase level generally stays elevated for up to two weeks, while the amylase level is elevated for up to five days
[2][18].
Additionally, a serum lipase test has a slightly higher sensitivity compared to the amylase test. At day 0–1 from the onset of symptoms, 100% is reached for lipase, while it is 95% for amylase. For day 2–3, the sensitivity ranges from 85%, whereas the specificity approximates 82% for lipase, in comparison to 68% for amylase. Based on the presented results, it can be concluded that lipase is particularly useful in the case of a delay between the time the patient seeks medical attention and onset of the symptoms
[2][18].
As reported by The American College of Gastroenterology in 2013, the measurement of both serum lipase and serum amylase does not demonstrate advantages in either treatment or profitability
[2][18]. Additionally, serum lipase has been found to be more sensitive than serum amylase among patients with acute pancreatitis secondary to alcohol abuse
[1][2][19]. Another research performed by Gwozdz et al.
[20], presents the diagnostic values of serum and urine enzyme assays in the recognition of AP. The study compares the diagnostic sensitivities of serum lipase, amylase, trypsinogen, elastase-1, the 2 h-timed urine amylase excretion, and the clearances of amylase and creatinine. All the serum tests showed the same sensitivity at the time of admission; however, in the following days, the serum lipase, trypsinogen, and elastase-1 tests presented considerably higher sensitivity than the serum amylase assay. During the second and following days, the diagnostic value of timed urine amylase excretion did not predominate over the serum amylase, and the ratio of amylase and creatinine clearances completely did not differ from each other
[20].
The study by Rompianesi et al.
[18], compared the diagnostic exactness of serum lipase, serum amylase, urinary amylase, and urinary trypsinogen-2 in the diagnosis of AP. Serum lipase and serum amylase, with a more than three times greater value than the standard threshold level, and urinary trypsinogen-2, with a value higher than the threshold of 50 ng/mL, seem to have similar sensitivities (79%, 72%, and 72%, respectively) and specificities (89%, 93%, and 90%, respectively). Researchers suggest that one of the above-mentioned parameters should have a low threshold, which would allow the initiation of treatment for AP even when the other parameters are normal. Additionally, they conclude that the occurrence of other disease entities should be considered, despite the incorrect results of the above-mentioned parameters, in order to avoid a misdiagnosis of AP
[17].
In conclusion, the biochemical diagnostic assay with a slightly greater clinical value is serum lipase. Lipase assays are nowadays instant, reliable, practical, more specific, and sensitive, and their price does not significantly exceed the price of amylase assays. The main advantages of serum lipase are the maintenance of elevated levels for a longer time in comparison with amylase, which is used in the case of the patients who initially present to the emergency department a few days after the onset of AP symptoms, and greater sensitivity in AP caused by alcohol overuse. The serum amylase assay is also performed in the diagnosis of AP, but it has a lower clinical value due to its greatest disadvantage, which is overall low specificity. A normal serum amylase should usually rule out the diagnosis of AP, except for AP secondary to hyperlipidemia and acute exacerbation of chronic pancreatitis, and when the assessment of amylase is delayed in the course of the disease. Nevertheless, the assessment of serum amylase has some advantages, such as inexpensiveness, ready availability, simple automated methods, and high sensitivity
[10][12][21]. The measurement of serum lipase is not affected by hypertriglyceridemia, but some drugs (for instance furosemide) give a possibility of increasing the serum activity
[22]. Other causes of increase are renal insufficiency, chronic pancreatitis, acute cholecystitis, or bowel obstruction
[18][22], whereas the measurement of serum amylase is competitively interfered with by hypertriglyceridemia; so, falsely low results can be produced, but it can be modified by using lipid clearing agents
[10][18]. Rarely seen abnormally low levels of amylase can occur during chronic pancreatitis, cystic fibrosis, smoking, obesity, and diabetes mellitus
[18].
To summarize, the crucial information concerning the serum lipase and amylase, have been presented in the table below (Table 2).
Table 2. Crucial information concerning indicator enzymes used in the diagnosis of acute pancreatitis, based on information published in the studies of Rompianesi et al., Matull et al., and Chase et al., as well as in other publications.
3.2.2. Urinary Trypsinogen-2
The sensitivity and specificity of the urinary trypsinogen-2 dipstick test are higher than those of the urinary amylase dipstick test
[23][24]. Higher levels in the urine than in the serum can occur because of the breakdown of the protein and the release of peptides during the increased proteolytic activity in AP and the consecutive decrease in the ability of the renal tubuli to reabsorb proteins
[25].
Urinary trypsinogen-2 remains increased for longer in patients with acute pancreatitis, compared to amylase, both in serum and urine
[25]. It is also better in the differentiation between severe and mild AP than the serum and urine amylase
[26].
A negative test excludes AP with a higher probability; therefore, it is more suitable for the screening of acute pancreatitis than serum lipase because of its higher sensitivity in comparison to serum lipase
[25][27].
Yasuda et al.
[28] confirmed that the rapid urinary trypsinogen-2 dipstick test and the levels of urinary trypsinogen-2 and TAP concentration may be considered as useful prognostic markers for the diagnosis of acute pancreatitis. The levels of urinary trypsinogen-2 TAP were considerably higher in patients with extended extra-pancreatic inflammation as evaluated by CT Grade, but not considerably higher in patients with hypo-enhanced pancreas lesions. Therefore, the measurement of urinary trypsinogen-2 and TAP could not select the patients who should have a CT examination
[28].
3.2.3. Urinary Amylase
The level of urinary amylase is measured by a clean-catch (midstream) urine sample or 24 h urine collection
[17]. Urinary amylase presents lower values of sensitivity (83%) and specificity (88%) than serum amylase (85% and 91%, respectively)
[25]. The urine test strips for amylase are considered to be useful bedside tests for the diagnosis of AP in patients with clinical pancreatitis
[29].
The use of the urine level of amylase (uAm) is limited in practice because the diagnostic ability of uAm is inferior to that of the serum level of amylase (sAm). uAm has been used as a marker after endoscopic retrograde cholangiopancreatography or pancreas transplantation. The amylase creatinine clearance ratio (ACCR) is an index that uses uAm. ACCR is known to increase during pancreatitis. However, it has little diagnostic value because of its low specificity and sensitivity. Terui et al.
[30] reported that the uAm/uCr ratio was correlated with sAm and may be an alternative to sAm for the prediction of hyperamylasemia. Furthermore, the correlation between sAm and uAm/uCr was low in babies and was significant in infants and schoolchildren. This indicated that the level of amylase itself cannot be used in babies. uAm/uCr could be appropriate for varied conditions of hyperamylasemia after the first year of life and does not appear to be influenced by elevated sCr. In the management of hyperamylasemia, uAm/uCr can potentially be used not for diagnosis but as a marker for following up on the levels of amylase. This result suggests the potential use of uAm/uCr as an alternative for sAm. Additionally, the use of urine samples results in a decreased need for blood sampling, which is especially beneficial in pediatric patients, and it reduces the risk of complications related to intravenous cannulation
[30].
3.3. Ultrasonography
Approximately 70–80% of cases of AP are caused by gallstones and alcohol abuse. Due to the distinctions in management, the differentiation of the above-mentioned etiologies is significant. Ultrasonography is the first and basic imaging test performed in all patients with suspected acute pancreatitis because of its accessibility and low expense and because it gives no exposure to radiation
[31]. This imaging modality is also used to diagnose acute biliary pancreatitis, excluding alcohol overuse as a main cause of AP. It allows the assessment of the condition of the biliary tract and the detection of biliary stones in the common bile duct (CBD).
The detection of gallstones by ultrasound has a sensitivity and specificity greater than 95%
[2][32][33][34][35][36][37]. Other studies report on sensitivity reaching approximately 92–96%
[38][39]. Nevertheless, ultrasound examinations are prone to some limitations that can be overcome by CT scans, such as the influence of intestinal gas occurring during ileus with bowel distension—a symptom commonly developing in the course of acute pancreatitis
[2][32][33][34][35][36][37]. There are the ultrasonographic examination methods, which are recommended for imaging the pancreas. These are the grayscale examination, harmonic imaging, color doppler, and the power or spectral doppler. The sections used to depict the pancreas are transverse and longitudinal upper epigastric sections and, particularly for the head and tail of the pancreas, oblique intercostal and subcostal sections. Depending on the position of the transducer, the pancreas can be examined through sections with different locations relative to the stomach
[40].
Specifically, positioning the transducer approximately halfway between the xiphoid appendix and the umbilicus provides examination through the transgastric or subgastric sections, while when the transducer is localized high in the epigastrum, the pancreas is visualized through the sections passing above the stomach antrum
[40].
However, high epigastric sections avoiding the colon (thus decreasing the risk of visualizing the intestinal gas) and transgastric sections, as well as sections using the left lobe of the liver as an acoustic window, are considered to be the best ultrasound windows that can be obtained during ultrasonographic examination of the pancreas
[40]. If the general condition of the patient with suspected AP allows it, the patient has to fast for at least 7–8 h, which is necessary to perform an appropriate ultrasound examination of the pancreas. The occurrence of food mass in the stomach might prevent the performance of precise and complete imaging of the pancreas and might also create images falsely suggesting pancreatic tumors
[40].
Therefore, if it is necessary to conduct a quick diagnosis without proper preparation of the patient, for instance when the management of the patient is urgent and has to be immediate, the images obtained with the use of ultrasound may be of poor quality and thus provide an uncertain diagnosis. As mentioned above, US of the abdomen provides detection of the gallstones with high sensitivity. However, the sensitivity decreases significantly, to the level of 65%, if the gallstones are localized in the infundibulum of the gallbladder or the diameter of the stones is less than 3 mm
[40].
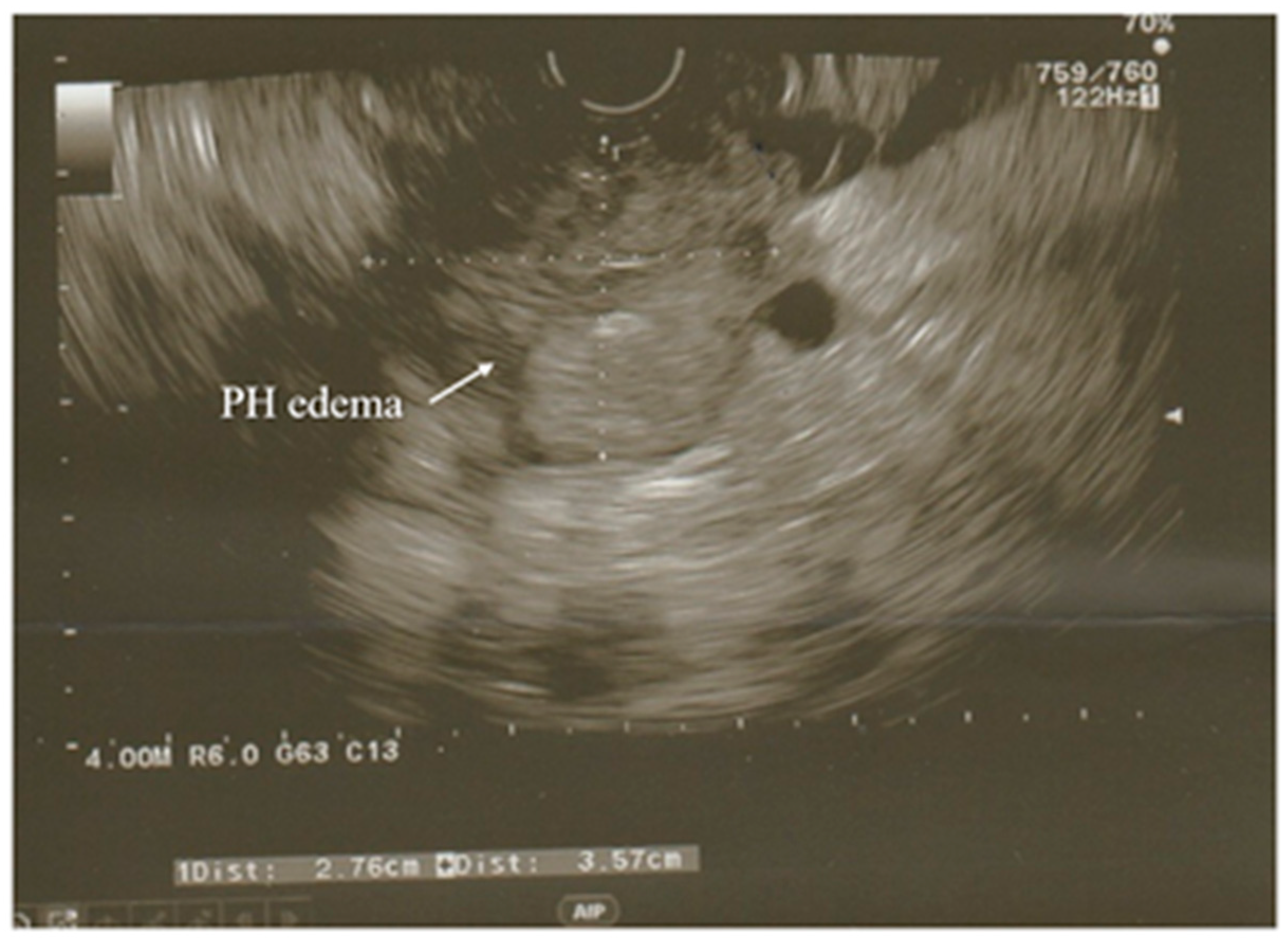
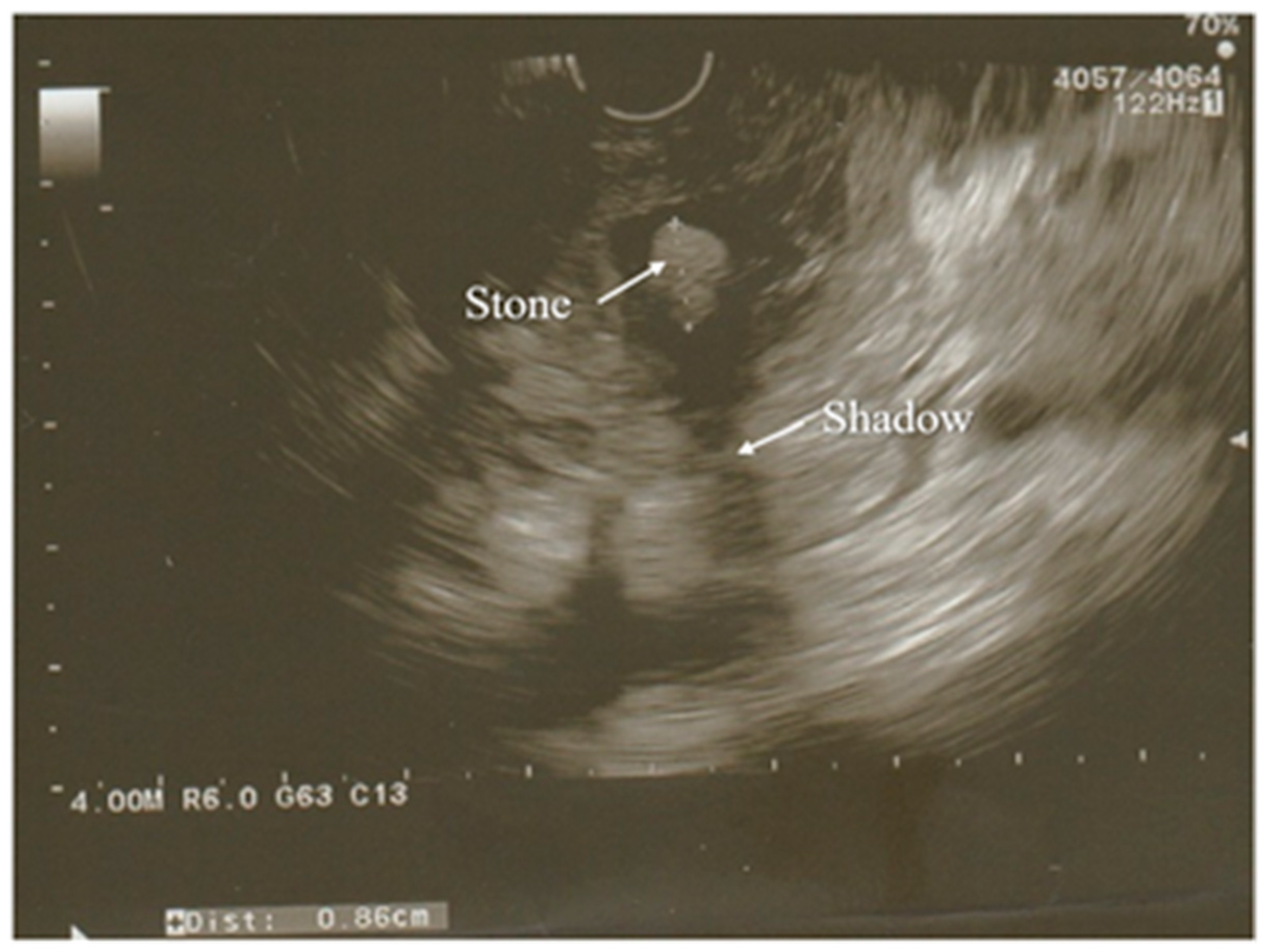
A modification of the standard ultrasound examination, with minimal invasiveness and high accuracy, used by choice to investigate the pancreas and biliary tract, is endoscopic ultrasound (EUS). Exemplary images from EUS examination have been presented on the
Figure 1 and
Figure 2. According to the results of the prospective study, EUS detects gallstones with a higher sensitivity in comparison to US (100% and 84%, respectively). Moreover, EUS is better than US in imaging the gallbladder because of the close proximity to the biliary system and, resulting from this, its high-image resolution
[38]. Similarly, EUS provides improved spatial resolution in comparison to the MRI and CT scan, also owing to the closeness of the EUS probe to the pancreas
[41]. In addition, EUS is a minimally invasive diagnostic modality and does not exhibit a comparatively high complication rate, unlike the ERCP. Both the EUS and the ERCP are characterized by the sensitivity reaching 97% for diagnosing choledocholithiasis. Due to this, EUS is helpful in the selection of the AP patients requiring therapeutic ERCP and thus avoiding the complications associated with diagnostic ERCP
[38][39][41]. Because of these advantages, EUS has become a valid imaging technique useful in the assessment of patients with pancreaticobiliary disease, including AP.
Figure 1. Acute pancreatitis with hypoechoic enlarged pancreatic head seen in EUS examination. PH—head of the pancreas.
Figure 2. Partially calcified gallstone (poor shadow behind it) was seen in the distal part of a common bile duct during the EUS examination. Surrounding pancreatic parenchyma is edematous.
In the case of AP, EUS is used to establish the cause of the disease after the decline of the acute attack of pancreatitis, while its usage during hospitalization for AP seems to be uncommon
[38][42].
One of the diagnostic challenges for gastroenterologists is idiopathic acute pancreatitis (IAP) and idiopathic recurrent acute pancreatitis (IRAP). If the initial evaluation allows the recognition of the etiology of pancreatitis, as happens in 70–90% of cases, the patient is diagnosed with AP/RAP, while in the remaining 10–30% of the patients the etiology cannot be identified after the initial evaluation, which enables AP/RAP to be defined as IAP/IRAP
[38][43][44]. The recognition of the etiology of pancreatitis is crucial for performing the appropriate evaluation, providing early treatment, and preventing relapse. Additionally, the determination of the etiology is important because 50% of untreated patients diagnosed with IRAP experience recurrent episodes that cause the progression of IRAP to CP
[38][43].
There are different explanations for the situations when the etiology of AP cannot be determined. These are, for instance, the occurrence of biological abnormalities in the first days of AP, which cause lipid- or calcium-metabolism disturbances to be difficult to diagnose; microlithiasis, which is difficult to diagnose by the use of standard imaging modalities; and the inflammation or necrosis of the pancreas, which may disturb imaging of pancreatic cystic or solid tumors
[38][45]. Management based on EUS is regarded as a reasonable approach for the assessment of patients with IAP/IRAP. The biliary tract disease is the most frequent diagnosis by EUS in IAP
[38]. The most significant indications for EUS are suspicion of gallstones in the CBD and/or gallbladder and microlithiasis, while the most valid indication for EUS in AP is the suspicion of acute biliary pancreatitis when transabdominal ultrasound and tomographic examinations fail to depict biliary calculi. Although EUS allows the imaging of the entire gallbladder, pancreas, and biliary ductal system in AP in most cases, patients with severe pancreatic necrosis, variations of gastroduodenal anatomy, or a rare location of the gallbladder may cause occasional difficulties in performing the examination by the usage of EUS
[38].
3.4. Magnetic Resonance Imaging
MRI and MRCP are used for the non-invasive evaluation of the pancreatic and biliary ducts, particularly the distal bile duct, which is hard to visualize by ultrasound, and are helpful in diagnosing the etiology of AP. MRI has many advantages, such as no exposure to radiation and the subsequent adverse effects on the human body; no use of a contrast agent in non-enhanced images; no premedication; no risk of developing complications; and the possibility to use it during an acute attack of pancreatitis and cholangitis, and it allows the visualization of the extraductal structures due to usage of standard T1-T2-weighted images. The non-enhanced MRI provides a clear presentation of the area of necrosis, and it is safe for the patients in the case of the impossibility of receiving iodinated contrast material due to kidney failure or allergies. Furthermore, MRI can visibly present local complications and stage the AP. Moreover, by the use of heavily T2-weighted sequences in non-enhanced MRCP, owing to high sensitivity to liquid, even a small amount of fluid in mild pancreatitis can be depicted
[42]. MRI, together with MRCP, is used to image noninvasively a few fat or necrotic materials localized in a fluid-filled lesion and pancreatic duct system, which in turn allows the assessment of the duct integrity and of whether the collections surrounding the pancreas are in communication with the pancreatic ducts
[36][37].
Sun et al.
[36] reported that MRI without enhancement is more precise and reliable in assessing the severity of AP in comparison with CT
[36]. Moreover, compared with CT, MRI is characterized by better soft-tissue contrast and the aforementioned no risk of radiation, which is significant for patients with AP requiring numerous follow-up examinations. Additionally, a non-enhanced MRI is a better radiologic modality in the diagnosis of mild AP as opposed to the CT
[36].
The diagnosis of AP by the usage of an MRI is dependent on the occurrence of morphologic and peripancreatic changes. An MRI allows a good performance of an examination detecting pancreatic necrosis and the complications of AP, such as abscesses, pseudocysts, or hemorrhage
[28]. Due to the increasingly common use of new MRI techniques that provide the diagnosis of pathological conditions associated with AP, the selection of management has improved. Additionally, multimodal MR images integrating a series of sequences, namely MRCP, T1- and T2-weighted imaging, dynamic contrast-enhanced (DCE) MR imaging, and diffusion-weighted imaging (DWI) with an apparent diffusion coefficient (ADC) map, are used more frequently for assessing AP before creating the plan of treatment
[42]. This imaging technique is recommended only for patients whose CBD is not appropriately visualized or present as normal features in an ultrasound examination and in whom an elevation of liver enzymes is detected.
The meta-analysis by Romagnuolo et al.
[46] presented the values of sensitivity and specificity of MRCP in the diagnosis of biliary obstruction, a probable pathomechanism of AP, which are, respectively, 95% and 97%. However, despite the advantages of MRCP, the cost of the examination limits its use in the diagnosis of gallstones, especially with the accessibility and usefulness of ultrasonography performed with the same objective
[2][47].
3.5. Computed Tomography
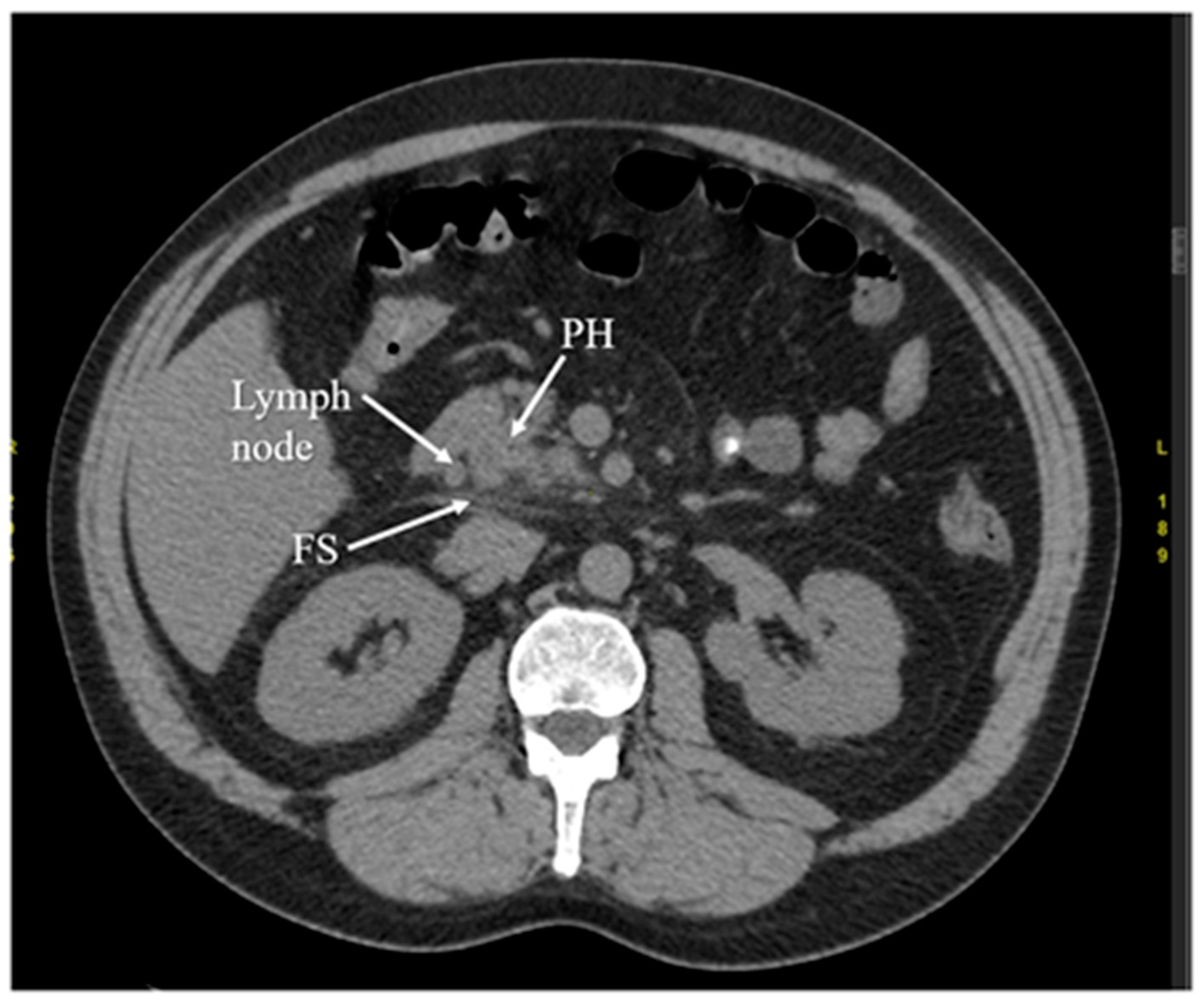
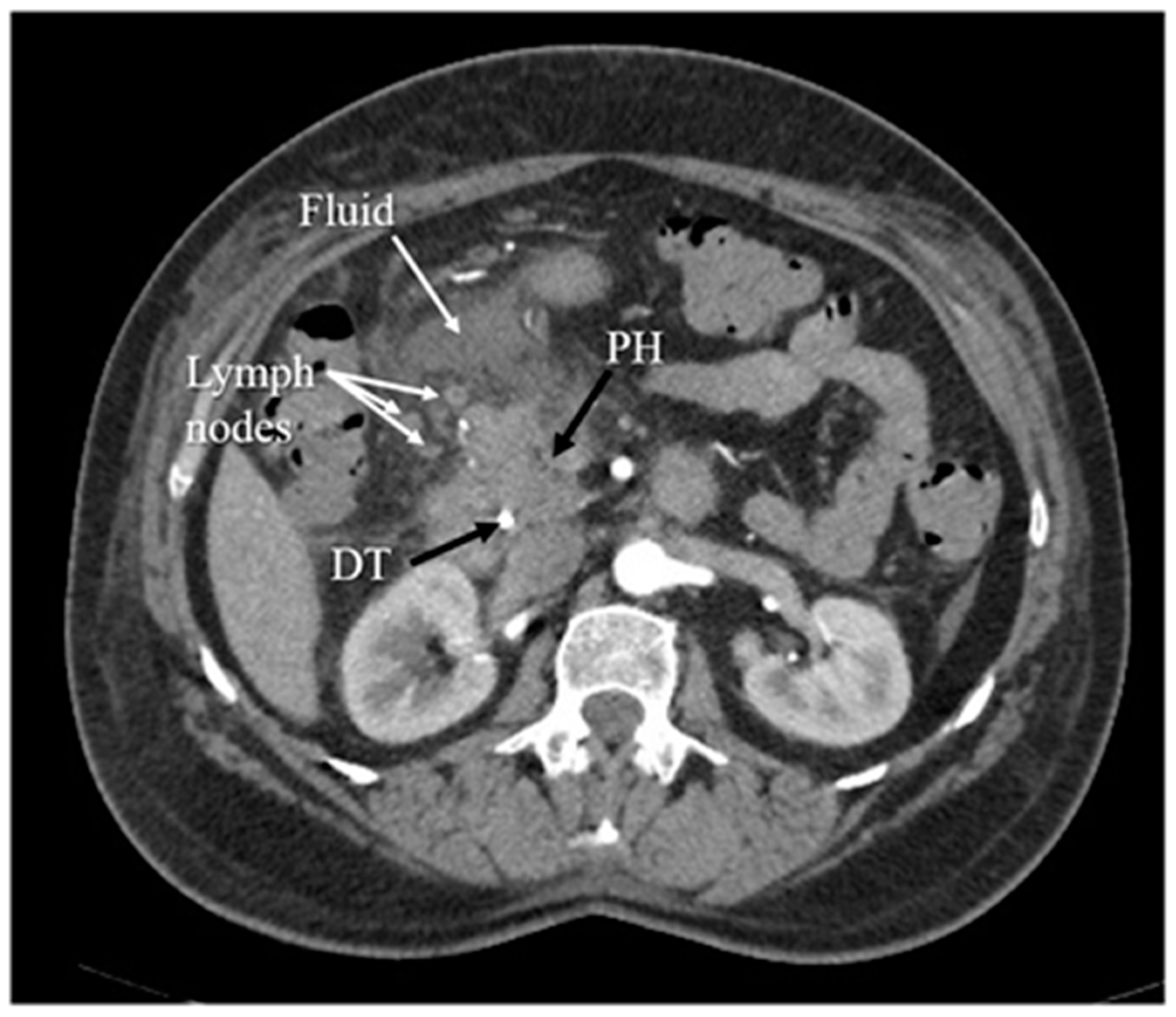
Another diagnostic method of acute pancreatitis is CT. Images obtained from CT have been shown in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. CT is performed in order to evaluate the extent of AP and to assess complications. It is also regarded as a splendid diagnostic method, characterized by fast scans with high spatial resolution, used for discovering the necrosis of the pancreas, presenting local complications, grading the acuity of inflammation, and assessing the severity of AP
[36][37][48]. CT also provides essential information for percutaneous management
[13]. It should be performed selectively in two cases. The first includes patients with suspected local complications of acute pancreatitis, such as signs of shock, peritonitis, or ambiguous ultrasound results, whereas the second case concerns patients with severe abdominal pain and extensive differential diagnosis, which confirms acute pancreatitis
[2]. In conclusion, each patient with abdominal pain and laboratory tests indicating AP should have a CT scan, in particular those patients with complications of AP or when the US examination is ambiguous.
Figure 3. Early phase of the acute pancreatitis with stranding of surrounding fat (FS) and single, enlarged lymph node. The arterial phase of CT. PH—head of the pancreas.
Figure 4. Inflammation of the head of the pancreas, with surrounding fluid and several enlarged lymph nodes. The arterial phase of CT. PH—head of the pancreas, DT—duodenal tube.
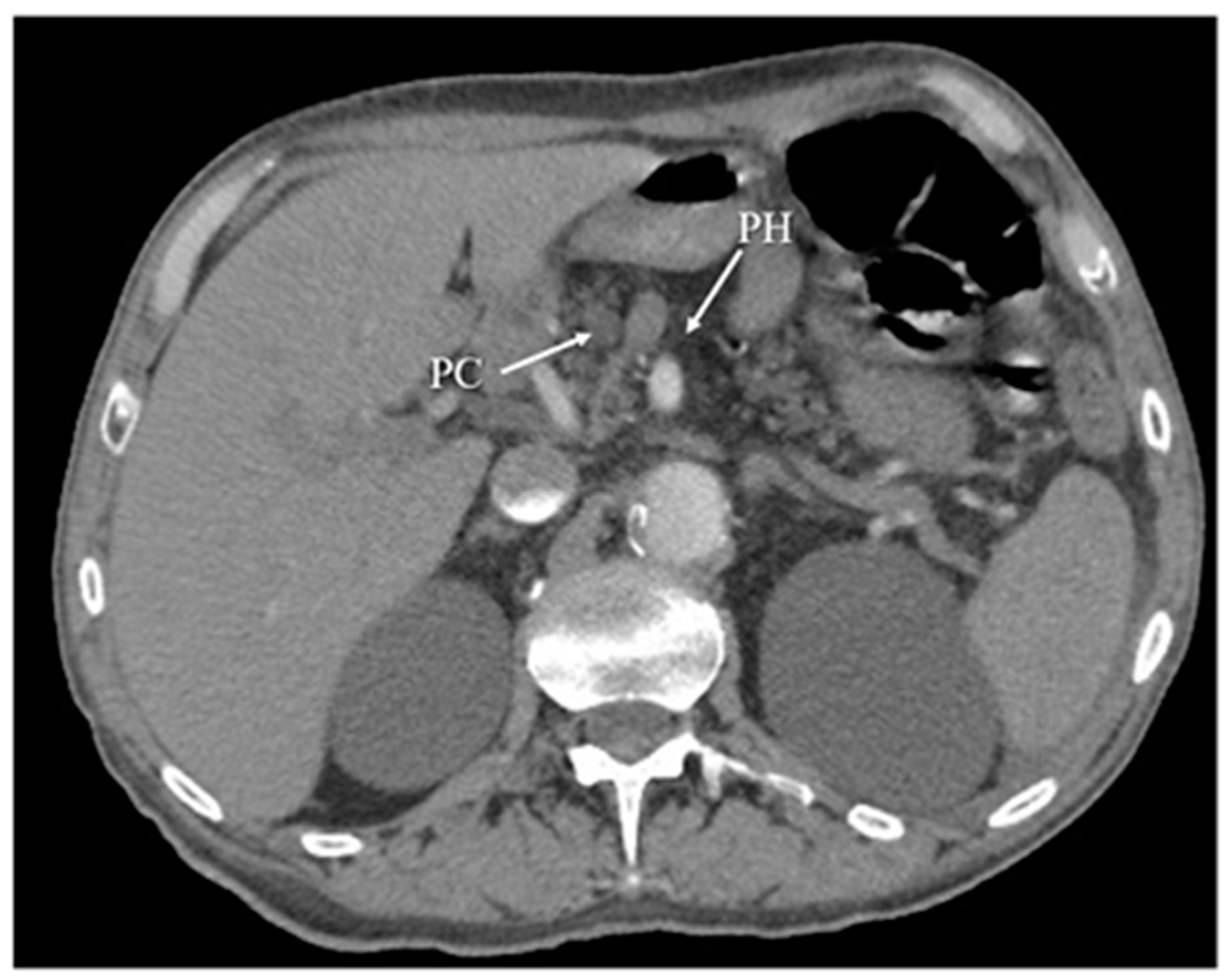
Figure 5. Chronic pancreatitis, with atrophy of pancreatic head (PH) parenchyma and pseudocyst (PC) in this region. The arterial phase of CT.
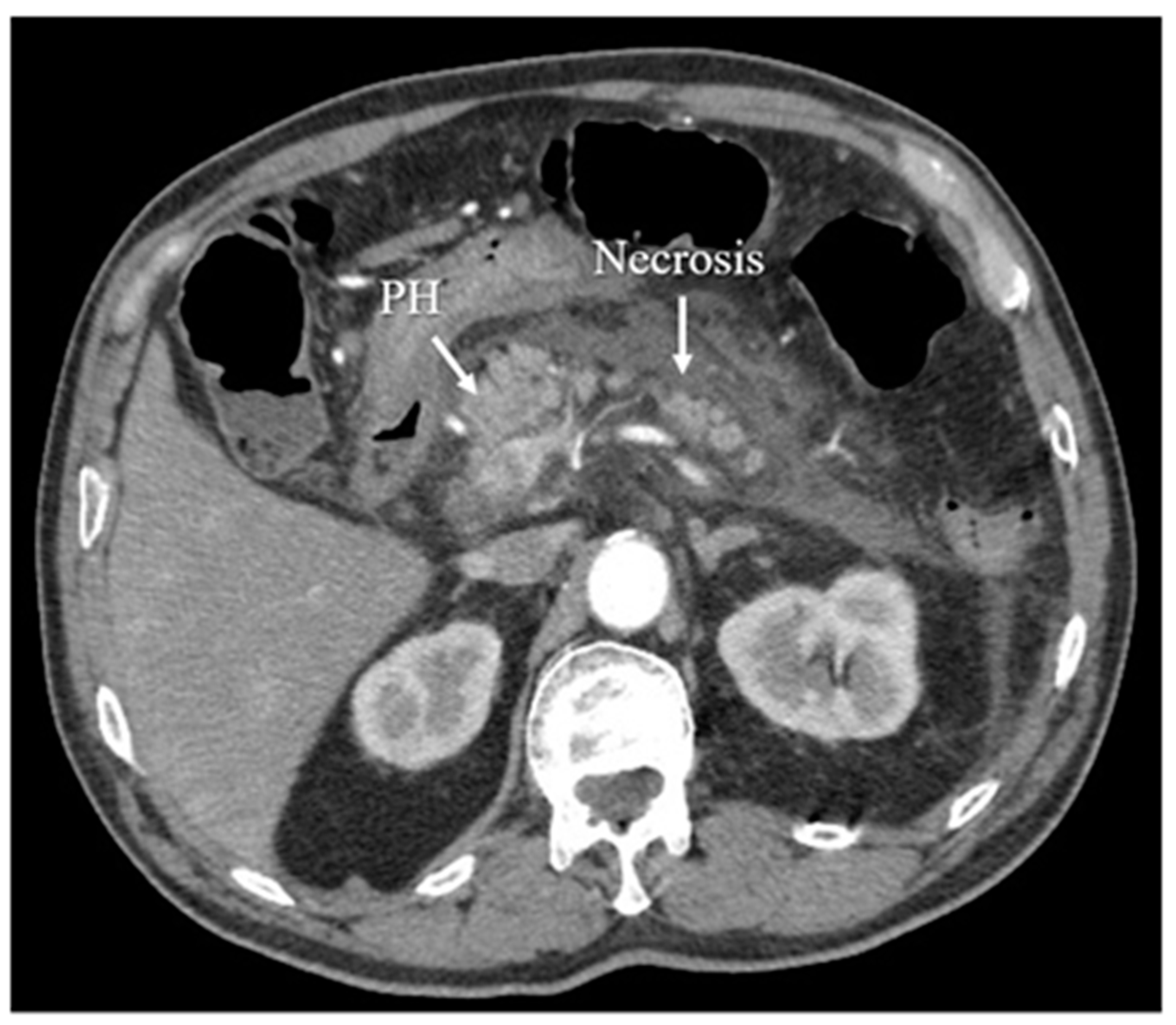
Figure 6. Acute necrotizing pancreatitis in the arterial phase of CT.
CT presents higher accuracy and sensitivity than US in diagnosing and providing the extent of the disease
[48]. However, it has disadvantages, such as a difficulty in distinguishing small quantities of necrotic or fat debris within one collection; it is also a potential radiation risk in the case of numerous follow-up scans
[36][37]. Computed tomography performed in order to diagnose local complications is most valuable 48–72 h after the onset of symptoms rather than at the time of admission. If the patient’s normovolemia has been restored and fluids have been resuscitated appropriately, in the absence of contraindications (e.g., renal failure), the patient should receive an intravenous contrast in order to evaluate for pancreatic necrosis.
In advanced cases, CT is used to exclude local complications and distinguish necrotizing acute pancreatitis and interstitial acute pancreatitis. In this case, computed tomography has a limited use during admission because the above-mentioned differentiation in acute pancreatitis is typically possible more than 3–4 days from the onset of symptoms. However, CT finds its use in early diagnosis in the case of the broad differential diagnosis that must be narrowed
[2][49][50]. According to the UK guideline for the management of acute pancreatitis, the only indications for the CT scan are inconclusive biochemical and clinical features
[51].
To conclude, researchers have presented the advantages and disadvantages of radiological tests used in acute pancreatitis in the Table 3.
Table 3. Summary of the radiological tests utilized in acute pancreatitis.
| Radiological Test |
Advantages |
Limitations |
| US |
|
-
Cannot be used to diagnose alcohol overuse as a main cause of AP;
-
Unfavorable influence of intestinal gas occurring during ileus with bowel distension on quality of imaging;
-
Adverse impact of food mass in the stomach on imaging of the pancreas—disruption of precision and completeness, creation of images falsely suggesting pancreatic tumors [32][35];
-
Poor quality, and therefore uncertain, diagnosis in the case of urgent management without proper preparation of the patient;
-
Significant decrease in the sensitivity of detection of the gallstones localized in the infundibulum of the gallbladder or characterized by the diameter less than 3 mm [40].
|
|
| EUS |
-
Minimal invasiveness;
-
Lower complication rate in comparison to ERCP—allows the avoidance of complications associated with diagnostic ERCP [38][39][41];
-
Detection of the gallstones with higher sensitivity in comparison to US;
-
Close proximity to the biliary system, allowing imaging of the gallbladder better than US and providing high-image resolution [39];
-
Improved spatial resolution in comparison to MRI and CT scan [41];
-
Regarded as a reasonable approach for assessment of patients with IAP/IRAP;
-
Alternative to transabdominal ultrasound and tomographic examinations in the case of unsuccessful imaging of biliary calculi;
-
Imaging of the entire gallbladder, pancreas, and biliary ductal system in AP in most cases [39].
|
|
|
| MRI |
-
The non-invasive evaluation of pancreatic and biliary ducts, particularly the distal bile duct, which is hard to visualize by ultrasound;
-
No exposure to radiation and subsequent adverse effects;
-
No use of a contrast agent in non-enhanced images;
-
Safe for the patients in the case of impossibility of receiving iodinated contrast material due to kidney failure or allergies;
-
No premedication;
-
No risk of developing complications;
-
Possibility to use during acute attack of pancreatitis and cholangitis;
-
Allows the visualization of the extraductal structures due to usage of standard T1-T2-weighted images;
-
Non-enhanced MRI provides clear presentation of the area of necrosis;
-
Visibly present local complications and stage the AP;
-
Allows the imaging of even a small amount of fluid in mild pancreatitis [42];
-
Used to image a few fat or necrotic materials localized in a fluid-filled lesion and pancreatic duct system, which in turn allows the assessment of the duct integrity and whether collections surrounding the pancreas are in communication with pancreatic ducts [36][37];
-
Non-enhanced MRI provides more precise and reliable image in assessing the severity of AP in comparison with CT;
-
Better soft-tissue contrast compared with CR;
-
Non-enhanced MRI is better in diagnosis of mild AP compared with CT [36];
-
Allows the detection of pancreatic necrosis and complications of AP, such as abscesses, pseudocysts, or hemorrhage [28];
-
High sensitivity and specificity of MRCP in the diagnosis of biliary obstruction [46].
|
-
The diagnosis of AP is dependent on the occurrence of morphologic and peripancreatic changes [28];
-
High cost of MRCP, which limits its use in the diagnosis of gallstones [2][47].
|
|
| CT |
-
Fast scans with high spatial resolution;
-
Allows the imaging of the necrosis of the pancreas and local complications of AP;
-
Enables the grading of the acuity of inflammation and the assessing of the severity of AP [36][37][48];
-
Provides essential information for percutaneous management [13];
-
High accuracy and sensitivity in diagnosing and providing the extent of the disease compared with US [48];
-
Used to exclude local complications and distinguish necrotizing acute pancreatitis and interstitial acute pancreatitis (more than 3–4 days from the onset of symptoms);
-
Used in early diagnosis, in the case of the broad differential diagnosis that must be narrowed [2][49][50].
|
|