Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Alginate (ALG), a naturally abundant linear and anionic polysaccharide, is generally obtained from the cell wall of brown seaweed belonging to the class Phaeophyceae, including Ascophyllum nodosum, Laminaria hyperborea, Laminaria digitata, Laminaria japonica, and Microcystis pyrifera, and many bacterial strains, including Acetobacter and Pseudomonas spp.
- alginate
- drug delivery system
- formulations
- administration route
1. Introduction
In the past, there had been a hurdle in the investigation to reveal naturally derived polymers with exceptional physicochemical characteristics and a high magnitude of compatibility for applications in drug delivery. Escorted by the advancement in pharmaceutical discovery methods in recent years, several therapeutically active substances have come to notice. Nevertheless, the curative agent’s delivery to the intentional site has been a severe hurdle in addressing many ailments. These novel pharmaceuticals need exquisite drug delivery systems (DDSs) that could be employed to improve their pharmacokinetics and pharmacodynamics characteristics, thereby advancing cell/tissue specificity along with their biocompatible properties. Therefore, the blooming of an effective DDS that can transport and administer an active accurately and safely to the desired site of action has to become the “bourne” of scientists.
A drug delivery system (DDS) refers to a system that carries curative substances inside the body to accomplish a required remedial outcome. Two principal classes of DDSs are identified, namely, conventional drug delivery systems and novel drug delivery systems (NDDSs) [1]. The actives are supplied by the conventional method via different routes such as oral, buccal/sublingual, rectal, intravenous, subcutaneous, and intramuscular. The conc. of therapeutic actives is not persistent during the therapy and demands continual dosage management in the conventional route [2]. Hence, this describes the prompt enhancement in the level of the drugs in the blood beyond the toxicity limit after individual administration and later declines to a sub-therapeutic level until the following administration [3]. The enhancement in actives conc. beyond the toxicity limit leads to perniciousness in the body. Moreover, the increase of repeated administration might sum up to the remedial non-compliance upon the sufferer [4].
To surmount the aforementioned limitations of the conventional approach, the progressive approach, NDDS, was prepared and included dosage forms. Consequently, the drug rate is sustained within the therapeutic-efficient level with controlled release of actives in both speed and period. Moreover, the NDDS transports actives to the particular action site with optimal dose and diminished toxic effect in contrast to the traditional drug delivery systems [4][5]. The convenient features of the NDDS (as pictured in Figure 1) encompass actives’ controlled release, the capability to utilize different administrative ways, improved active guard and efficiency, the improved substrate solubility showing low solubility, and a novel business market prospective to retrieve pharmaceuticals that have been unsuccessful throughout the traditional drug delivery approaches [1][6][7].
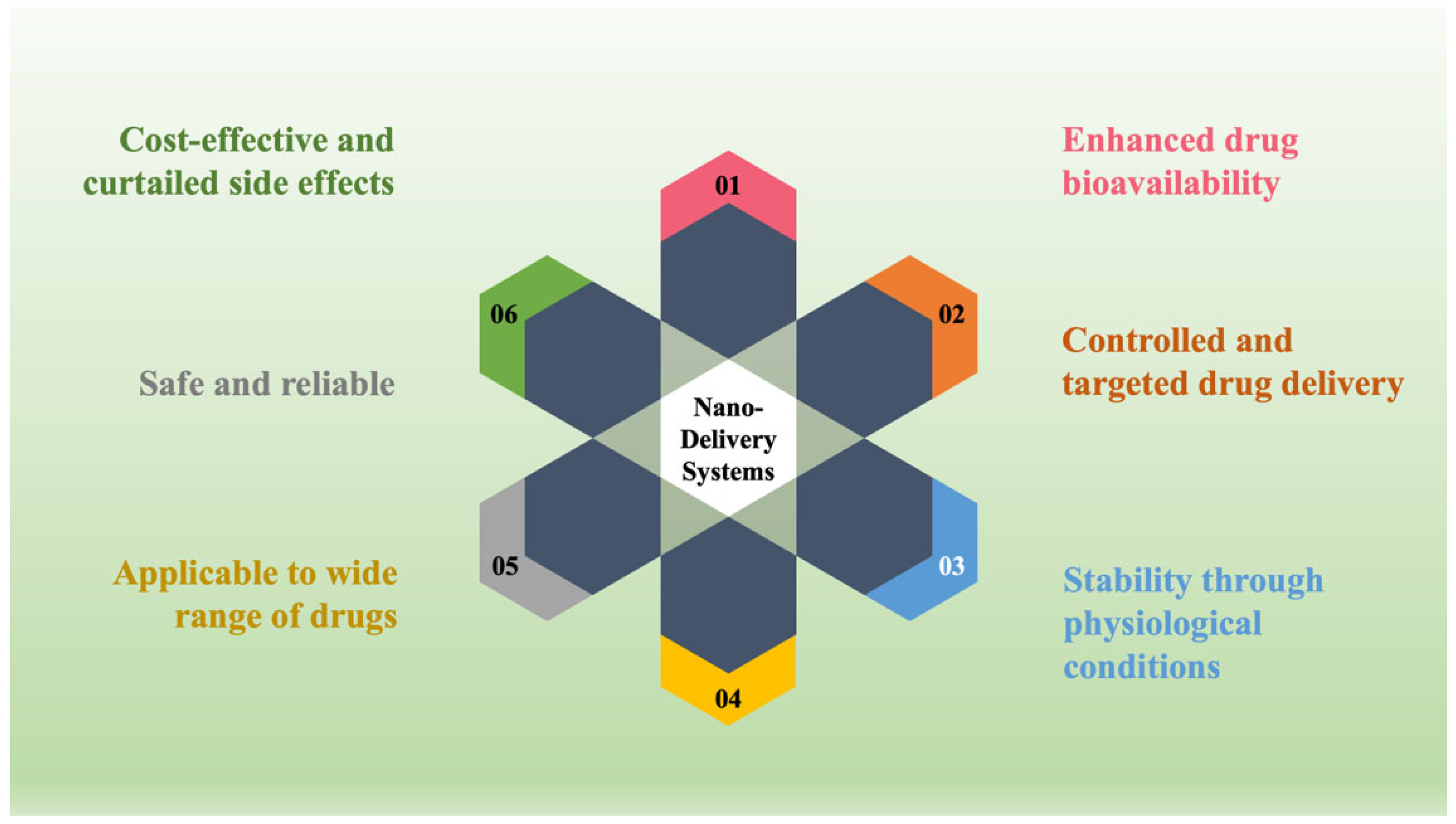
Figure 1. The ideal characteristics of nano delivery systems.
Out of diverse mechanisms of delivery, “controlled drug delivery” and “targeted drug delivery” have been sighted as some of the utmost challenging and fast-progressing investigational areas in the past four years. It provides myriad benefits in contrast to conventional systems, e.g., it improves the absorption rate and biocompatible properties, enhances the actives protection against proteolytic enzyme degradation, cell and tissue-specific active targeting, and helps to regulate active levels within the body, inside the therapeutic level range, over a more prolonged time [8][9]. However, despite the advantages of controlled releases that were formerly persuasive, potential shortcomings, for example, toxicity inside the body, complicated synthetic pathways and the resulting degradation by-products, and operative methods required to explant systems that are non-biodegradable, persist as severe impediments [10][11].
In NDDSs, the active carrier is a base that permits actives to be carried to the intended location, delivering the actives in a controlled manner, thereby enhancing the active bioavailability [12]. Nanoparticles, liposomes, microspheres, polymeric micelles, etc., are some of the significant actives carriers utilized in NDDSs [13][14][15][16][17][18].
Nonetheless, due to their suitable, variable characteristics, polymeric biomaterials are the most alluring opportunity for delivering drugs in a controlled and directed manner. They can be produced on an industrial scale and readily customized to meet the required applications [19]. But the polymer selection utilized for the drug carrier preparation performs a critical function in the process of actives delivery. The two kinds of polymers that are obtainable in the market are natural and synthetic polymers. Natural polymers (for example, chitosan, alginate, and bacterial cellulose), as well as many synthetic polymers such as poly(lactic-co-glycolic acid) (PLGA), poly-L-lysine (PLL), polycaprolactone (PCL), etc., are used as carriers for drug delivery. These polymers have less toxicity, are biocompatible, and are biodegradable, by which they are degraded via the action of enzymes [20][21].
2. Sources of Extraction and Properties of Alginate
ALG, a naturally abundant linear and anionic polysaccharide, is generally obtained from the cell wall of brown seaweed belonging to the class Phaeophyceae [22], including Ascophyllum nodosum, Laminaria hyperborea, Laminaria digitata, Laminaria japonica, and Microcystis pyrifera [23], and many bacterial strains, including Acetobacter and Pseudomonas spp. Although it can be created from bacterial origins, it is commercially accessible from algae as SA in its salt form [24]. They are a class of linearly arranged biopolymers comprising 1,4-linked-β-D-mannuronic acid (M-blocks) and 1,4-α-L-guluronic acid (G-blocks) residues ordered in sequences of identical (MM, GG) or heterogeneous (MG) blocks (as portrayed in Figure 2) [25]. Divalent cations, for example, Ba2+ and Ca2+, can rapidly construct egg-box systems with G block to build ALG hydrogels via the procedure of gelation [26]. Increasing the molecular weight and G-block length dramatically increases the mechanical properties of ALG. Commercially available ALG has an average molecular weight varying between 32,000 and 400,000 g/mol. The ALG solutions have a maximum viscosity at pH 3.0–3.5 because of the hydrogen bonding of the carboxylate groups forming the ALG backbone [23].
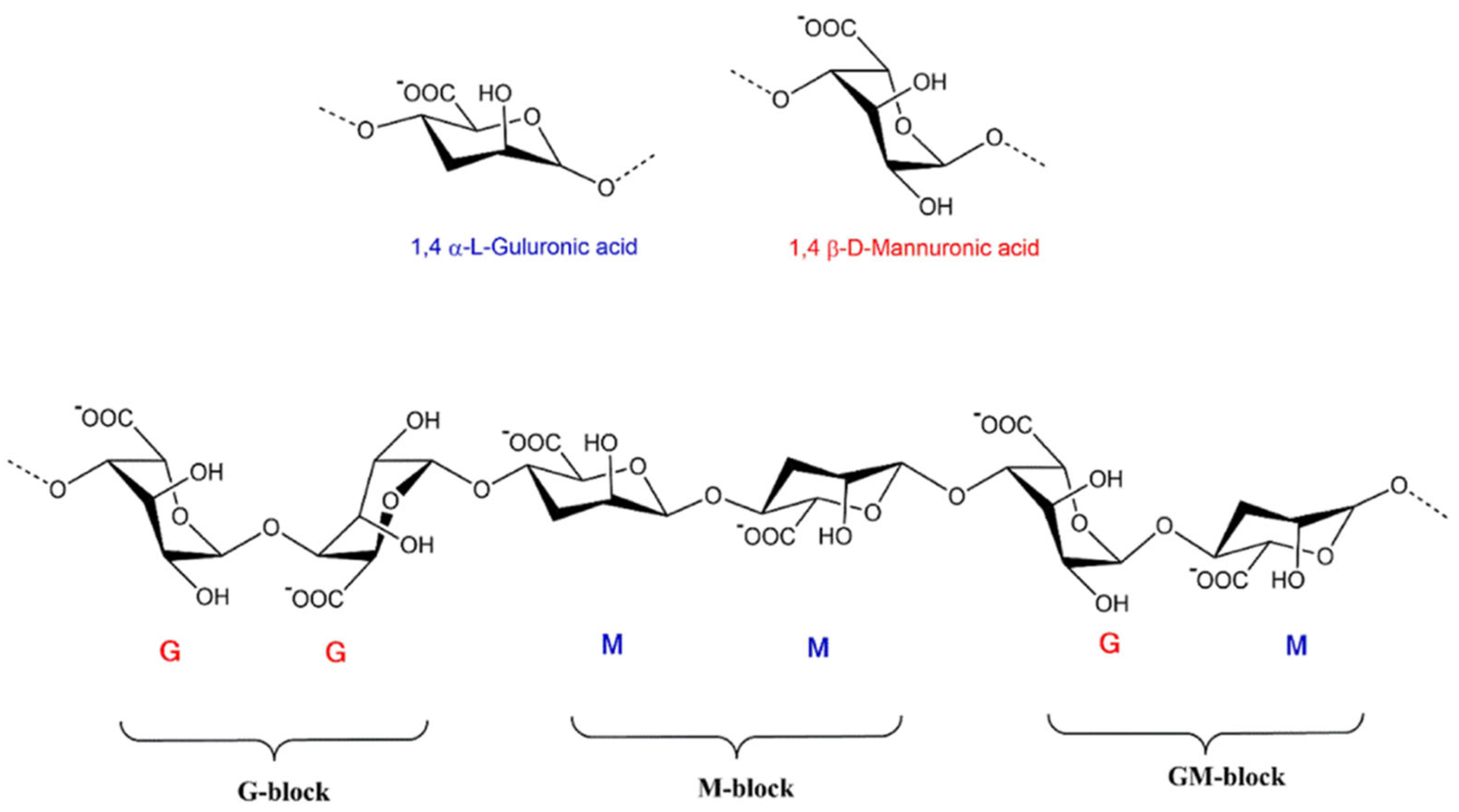
Figure 2. The monomer’s conformation and blocks distribution of ALG salt [27].
Recently, a multistage extraction process (from brown seaweeds, as illustrated in Figure 3) is being carried out, which involves acid pretreatment of the seaweed extract, followed by aqueous alkali treatment (mainly sodium hydroxide) in which different salt forms of natural ALG are modified into aqueous-soluble SA [28]. Subsequently, the filtered extract is incorporated with sodium or calcium chloride, and ALG gets precipitated. Then, dilute HCL is added, and salts of ALG get converted to alginic acid; following further purification and modification, a powder form of water-soluble SA is prepared [29].
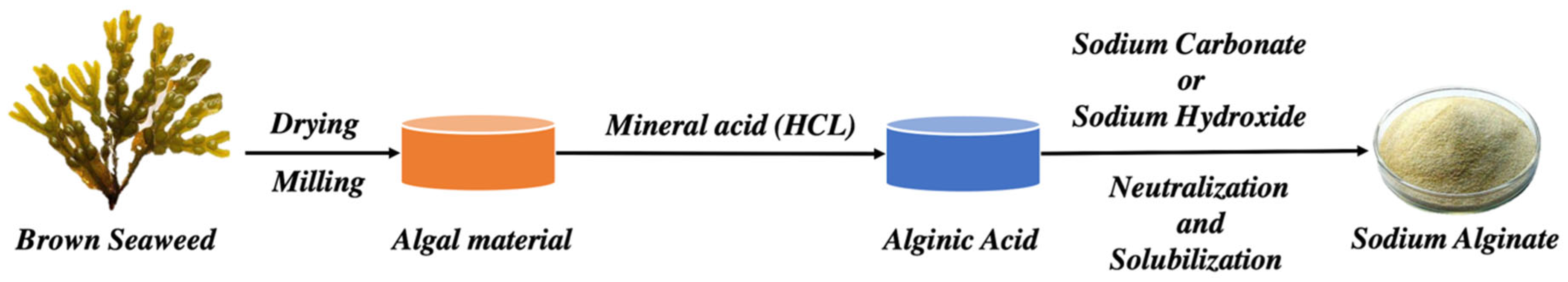
Figure 3. The extraction technique of ALG from brown seaweeds.
The ALG production from bacterial biosynthesis has definite physical characteristics and chemical structures different from the extracted ALG from brown seaweed. The different steps of ALG biosynthesis are (1) synthesis of the precursor substrate, (2) transfer polymerization and cytoplasmic membrane, (3) transport and alteration of the periplasmic membrane, and (4) conveying via the outer membrane [30].
Table 1 denotes the literature survey of various extraction methods of ALG.
Table 1. Various methods for the extraction of ALG.
| Seaweed Species | Components Used for Extraction | Extraction Yield (% Dry Weight—d.w.) |
Characteristics | References |
|---|---|---|---|---|
| Sargassum mangarevense and Turbinaria ornata | Formaldehyde-acidification-Na2CO3-ethanol | Sargassum mangarevense (6.0–12.4% d.w.); T. ornata (16.8–21.1% d.w.) | T. ornata demonstrated a greater viscosity and yield than S. mangarevense. Both the species showed a high M:G ratio (1.25–1.42) compared to the reported literature. No seasonal variation was observed. | [31] |
| Sargassum vulgare | Formaldehyde-HCl-Na2CO3 | 16.9% | M/G ratio for S. vulgaris low density and S. vulgaris high density were higher than most Sargassum species ALG (1.56 and 1.27, respectively). Optimal conditions for extraction of ALG from S. vulgaris were 60 °C and 5 h duration. Newtonian activity seen for a solution fraction was 0.7% for SVLV, whereas, it was 0.5%. for the SVHV sample. | [32] |
| Sargassum turbinarioides Grunow | Formaldehyde-HCl-Na2CO3 | 10% | M/G ratio was 0.94, η < 1, M.W. (5.528 × 105 g mol−1), and polydispersity index were low (1.43). | [33] |
| Laminaria digitata and Ascophyllum nodosum | Na2CO3 or NaOH after different acid pre-treatments (H2SO4 and HCl) at different temperatures | 28.65 ± 0.92% to 78.02 ± 16.81% | Unrefined extracts produced films with appropriate mechanical characteristics without cationic complexation. The treatment with sodium carbonate resulted in extracts with good plasticizing capacity, while sodium hydroxide extraction guided to polymer chains with enhanced cross-linking ability. Ascophyllum films possessed radical scavenging property. | [34] |
| Tunisian seaweed (Cystoseira barbata) | high-temperature alkaline extraction | 9.9% | M/G ratio was 0.59, pseudoplastic flow behavior. The emulsion formed was highly stable at acidic pH and less affected by temperature. CBSA exhibited DPPH radical scavenging activity (74% 33 inhibition at a concentration of 0.5 mg/mL). Excellent hydroxyl-radical scavenging activity, ferric reducing potential, and protection against DNA breakage were observed. | [35] |
| Macrocystis pyrifera | Ethanol route, HCl route, CaCl2 route | 25–33% | Direct polymer precipitation with ethanol gave the best yield. The precipitation step with calcium and cation exchange gave an ALG with poor viscoelastic properties. A pH higher than 3.5 in the acid pre-treatment step amended the ethanol route, thereby preventing the ethanol linkages from being ruptured. | [36] |
| Sargassum muticum | conventional alkaline extraction and hydrothermal fractionation | 5.04–10.09% | EC50 values for DPPH radical scavenging (0.72 and 1.18 g L−1 at 190 °C than at 220 °C, respectively) were comparable with synthetic antioxidants. However, at the minimum tested value (0.2 g L−1), the manufactured extracts at 190 °C appeared to be prooxidant. The AAC values that reached the maximum tested concentration at (0.5 g L−1) were similar to those for BHA and BHT. | [37] |
| Sargassum sp. (SRG) (genus Sargassum), Turbinaria sp. (TRB) (genus Turbinaria), Hormophysa sp. (RHT) (genus Hormophysa) | HCl-Na2CO3-EDTA | SRG 31 RHT 31 TRB 30 |
M/G ratio by 1H NMR 0.7–1.0, while after hydrolysis was 0.52–1.1. TRG with M/G <1 Gave a softer gel than SRG, while RHT, rich in mannuronic acid, gave the softest gel. | [38] |
| Sargassum muticum | Formaldehyde-HCl-Na2CO3 | 13.57 ± 0.13% | Optimum conditions for extraction are 86 °C temperature, 3% alkali, and 93% ethanol for 3 h. M/G was 1.08. | [39] |
ALG’s biocompatibility, rheological properties [40][41], biodegradability, marginal toxicity, and chemical versatility [42] are prominent, along with its exceptional characteristics in producing stable gel in aqueous conditions and a mild environment by adding multivalent cations, making ALG beneficial for drug delivery [43][44]. Furthermore, ALGs can be readily created into various semi-solid or solid frameworks under a moderate environment due to their exceptional sol/gel transition capability. Hence, ALGs are also frequently utilized as viscosity-enhancing substances and thickening agents in the pharma industry [44].
The percentage of the three sorts of blocks—MM, GG, and MG—specifies the ALG’s physical properties. In having a higher percentage of G, ALGs have higher gelling characteristics, while in having a high M content, ALGs have greater viscosity. Determining the M/G ratio is also essential for ALGs, those with a high ratio of M/G produce elastic gels, while those with small M/G ratios produce brittle gels [33][45]. The ALG-based formulation’s mechanical characteristics rigorously rely on the count and conc. of G and M units. If G residues beat M, the formulation exhibits higher mechanical rigidity. Thus, by changing the content of G and M, it is possible to modify the elastic modulus [46].
ALG undergoes hydration at low pH, which leads to the development of “acid gels”, which are highly viscous. The pH sensitivity of ALG can be attributed to acidic pendant groups that accept or release protons due to intermolecular binding when the pH is changed. As a result, the water molecules enter the ALG matrix and get physically entrapped within them but are still free to migrate. This ability is essential in the formation of ALG gels for cell encapsulation [47]. The ALG’s capability to produce two different classes depending on the pH, i.e., acid gel at low pH and ionotropic gel at higher pH, makes it unique compared to neutral molecules [48].
ALG has excellent mucoadhesive characteristics due to the existence of free carboxyl moieties, enabling the biopolymer to attach to mucin via hydrogen bonding as well as electrostatic interaction. On the other hand, ALG solubility is largely dependent on environmental pH and, accordingly, affects their mucoadhesive property since only ionized carboxyl groups are proficient in engaging with tissues of the mucosa. Moreover, soluble ALG assists the penetration of solvent through the polymer matrix, forming a highly viscous and cohesive gel framework for enhancing the mucoadhesive bond strength. In contrast, excessive and exorbitant ALG matrix hydration in physiological solution could diminish mucoadhesive properties due to the weakening of ALG functional groups accessible for mucosal tissue interactions [49][50].
ALGs can be tailored to fulfill the requirements of either pharmaceutical or biomedical applications. Owing to their high water uptake, sustained release, enhanced porosity, and non-immunogenicity, ALGs have found widespread applications in wound dressings [51]. ALG-based composites offer great utility in bioremediation by removing heavy metals, dyes, antibiotics, and other contaminants from wastewater [52]. Based on the types of cross-linkers and the cross-linking approaches used, materials ranging from small drug substances to macromolecular proteins can be designed as controlled drug delivery systems [53].
Biocompatibility is one more vital factor to be studied as the extraction of ALG obtained from nature is accompanied by the existence of numerous impurities capable of inciting allergic responses. In effect, an immune reaction has been stated in industrial-grade ALG; nonetheless, the multi-stage extraction method for eliminating metallic impurities and polyphenolic substances permits the acquisition of ALG of substantially high purity for use in biomedical applications [54].
ALG’s antioxidant and anti-inflammatory actions have also been noticed. It has been reported that ALG oligosaccharides reduce nitric oxide, reactive oxygen species (ROS), and eicosanoids, such as prostaglandin E2 and cyclooxygenase COX-2 production [55][56][57]. Thus, ALG’s exceptional characteristics have unlocked the doorways in its widespread actives delivery applications.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169035
References
- Jain, K.K. Drug Delivery Systems—An Overview. Methods Mol. Biol. 2008, 437, 1–50.
- Ansari, M.J.; Ahmed, M.M.; Anwer, M.K.; Jamil, S.; Alshetaili, A.S.; Ali, R.; Shakeel, F. Formulation and Characterization of Fluconazole Loaded Olive Oil Nanoemulsions. Indo Am. J. Pharm. Sci. 2017, 4, 852–860.
- Agrawal, P. Significance of Polymers in Drug Delivery System. J. Pharmacovigil. 2015, 3, 10–12.
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.P.S. Controlled Release Drug Delivery Systems. Pharma Innov. J. 2012, 1, 24–32.
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an Emerging Platform for Drug Delivery: A State-of-the-Art Review. J. Mater. Chem. B 2022, 10, 2781–2819.
- Ansari, M.J. An Overview of Techniques for Multifold Enhancement in Solubility of Poorly Soluble Drugs. Curr. Issues Pharm. Med. Sci. 2019, 32, 203–209.
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and Characterization of Ethyl Cellulose Nanosponges for Sustained Release of Brigatinib for the Treatment of Non-Small Cell Lung Cancer. J. Polym. Eng. 2020, 40, 823–832.
- Grund, S.; Bauer, M.; Fischer, D. Polymers in Drug Delivery-State of the Art and Future Trends. Adv. Eng. Mater. 2011, 13, 61–87.
- Zhuo, F.; Abourehab, M.A.S.; Hussain, Z. Hyaluronic Acid Decorated Tacrolimus-Loaded Nanoparticles: Efficient Approach to Maximize Dermal Targeting and Anti-Dermatitis Efficacy. Carbohydr. Polym. 2018, 197, 478–489.
- Langer, R. Polymeric Delivery Systems for Controlled Drug Release. Chem. Eng. Commun. 1980, 6, 1–48.
- Mady, F.M.; Ibrahim, S.; Abourehab, M. Development and Evaluation of Alginate-Gum Blend Mucoadhesive Microspheres for Controlled Release of Metformin Hydrochloride. J. Adv. Biomed. Pharm. Sci. 2021, 4, 111–118.
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell Membrane Cloaked Nanomedicines for Bio-Imaging and Immunotherapy of Cancer: Improved Pharmacokinetics, Cell Internalization and Anticancer Efficacy. J. Control. Release 2021, 335, 130–157.
- Pramanik, S.; Sali, V. Connecting the Dots in Drug Delivery: A Tour d’horizon of Chitosan-Based Nanocarriers System. Int. J. Biol. Macromol. 2021, 169, 103–121.
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-Assembled Biodegradable Polymeric Micelles to Improve Dapoxetine Delivery across the Blood-Brain Barrier. Int. J. Nanomed. 2018, 13, 3679–3687.
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and Development of Novel Hyaluronate-Modified Nanoparticles for Combo-Delivery of Curcumin and Alendronate: Fabrication, Characterization, and Cellular and Molecular Evidences of Enhanced Bone Regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281.
- Fatima, I.; Rasul, A.; Shah, S.; Saadullah, M.; Islam, N.; Khames, A.; Salawi, A.; Ahmed, M.M.; Almoshari, Y.; Abbas, G.; et al. Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections. Molecules 2022, 27, 2936.
- Ashfaq, M.; Shah, S.; Rasul, A.; Hanif, M.; Khan, H.U.; Khames, A.; Abdelgawad, M.A.; Ghoneim, M.M.; Ali, M.Y.; Abourehab, M.A.S.; et al. Enhancement of the Solubility and Bioavailability of Pitavastatin through a Self-Nanoemulsifying Drug Delivery System (SNEDDS). Pharmaceutics 2022, 14, 482.
- Abdel-Kader, M.; Al-Shdefat, R. Evaluation of Antifungal Activity of Olive Oil Based Nanoemulsions. Bull. Env. Pharmacol. Life Sci. 2016, 5, 1–4.
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11.
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polym. Rev. 2015, 55, 371–406.
- Pramanik, S.; Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A.S. Bacterial Cellulose as a Potential Biopolymer in Biomedical Applications: A State-of-the-Art Review. J. Mater. Chem. B 2022, 10, 3199–3241.
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial Alginate Production, Modification and Its Applications. Microb. Biotechnol. 2013, 6, 637–650.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Remminghorst, U.; Rehm, B.H.A. Bacterial Alginates: From Biosynthesis to Applications. Biotechnol. Lett. 2006, 28, 1701–1712.
- Sachan, N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium Alginate: The Wonder Polymer for Controlled Drug Delivery. J. Pharm. Res. 2009, 2, 1191–1199.
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen Quantitatively Monitoring the Release of Ca2+ during Swelling and Degradation Process in Alginate Hydrogels. Mater. Sci. Eng. C 2019, 104, 109951.
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8.
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.Y.; Ward, K. Multistage Extraction and Purification of Waste Sargassum Natans to Produce Sodium Alginate: An Optimization Approach. Carbohydr. Polym. 2018, 198, 109–118.
- Qin, Y. Alginate Fibres: An Overview of the Production Processes and Applications in Wound Management. Polym. Int. 2008, 57, 171–180.
- Satheeshababu, B.K.; Mohamed, I. Synthesis and Characterization of Sodium Alginate Conjugate and Study of Effect of Conjugation on Drug Release from Matrix Tablet. Indian J. Pharm. Sci. 2015, 77, 579–585.
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, Mannitol, Phenolic Compounds and Biological Activities of Two Range-Extending Brown Algae, Sargassum Mangarevense and Turbinaria Ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043.
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and Physicochemical Characterization of Sargassum Vulgare Alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074.
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum Turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137.
- Blanco-Pascual, N.; Montero, M.P.; Gómez-Guillén, M.C. Antioxidant Film Development from Unrefined Extracts of Brown Seaweeds Laminaria Digitata and Ascophyllum Nodosum. Food Hydrocoll. 2014, 37, 100–110.
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367.
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the Extraction-Purification Conditions on Final Properties of Alginates Obtained from Brown Algae (Macrocystis Pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371.
- González-lópez, N.; Moure, A.; Domínguez, H. Hydrothermal Fractionation of Sargassum Muticum Biomass. J. Appl. Phycol. 2012, 24, 1569–1578.
- Pascaline, M.; Andriamanantoanina, H.; Heyraud, A.; Rinaudo, M. Food Hydrocolloids Structure and Properties of Three Alginates from Madagascar Seacoast Algae. Food Hydrocoll. 2013, 32, 143–146.
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of Alginate from Sargassum Muticum: Process Optimization and Study of Its Functional Activities. J. Appl. Phycol. 2016, 28, 3625–3634.
- Vauchel, P.; Arhaliass, A.; Legrand, J.; Kaas, R.; Baron, R. Decrease in Dynamic Viscosity and Average Molecular Weight of Alginate from Laminaria Digitata during Alkaline Extraction1. J. Phycol. 2008, 44, 515–517.
- Vauchel, P.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. A New Process for Extracting Alginates from Laminaria Digitata: Reactive Extrusion. Food Bioprocess Technol. 2008, 1, 297–300.
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 27–66. ISBN 978-981-10-6910-9.
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152.
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34.
- Fertah, M.; Belfkira, A.; montassir Dahmane, E.; Taourirte, M.; Brouillette, F. Extraction and Characterization of Sodium Alginate from Moroccan Laminaria Digitata Brown Seaweed. Arab. J. Chem. 2017, 10, S3707–S3714.
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633.
- Jain, D.; Bar-Shalom, D. Alginate Drug Delivery Systems: Application in Context of Pharmaceutical and Biomedical Research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584.
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630.
- Haugstad, K.E.; Håti, A.G.; Nordgård, C.T.; Adl, P.S.; Maurstad, G.; Sletmoen, M.; Draget, K.I.; Dias, R.S.; Stokke, B.T. Direct Determination of Chitosan–Mucin Interactions Using a Single-Molecule Strategy: Comparison to Alginate–Mucin Interactions. Polymers 2015, 7, 161–185.
- Taylor, C.; Pearson, J.P.; Draget, K.I.; Dettmar, P.W.; Smidsrød, O. Rheological Characterisation of Mixed Gels of Mucin and Alginate. Carbohydr. Polym. 2005, 59, 189–195.
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42.
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B.; et al. Technology Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356.
- Pawar, S.N.; Edgar, K.J. Biomaterials Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305.
- Spasojevic, M.; Bhujbal, S.; Paredes, G.; de Haan, B.J.; Schouten, A.J.; de Vos, P. Considerations in Binding Diblock Copolymers on Hydrophilic Alginate Beads for Providing an Immunoprotective Membrane. J. Biomed. Mater. Res. Part. A 2014, 102, 1887–1896.
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-Inflammatory Activity of Guluronate Oligosaccharides Obtained by Oxidative Degradation from Alginate in Lipopolysaccharide-Activated Murine Macrophage RAW 264.7 Cells. J. Agric. Food Chem. 2015, 63, 160–168.
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant Activities of Sulfated Polysaccharides from Brown and Red Seaweeds. J. Appl. Phycol. 2007, 19, 153–160.
- Maciel, D.; Figueira, P.; Xiao, S.; Hu, D.; Shi, X.; Rodrigues, J.; Tomás, H.; Li, Y. Redox-Responsive Alginate Nanogels with Enhanced Anticancer Cytotoxicity. Biomacromolecules 2013, 14, 3140–3146.
This entry is offline, you can click here to edit this entry!
