Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Fresh-cut fruits have been in great demand by consumers owing to the convenience of buying them in shopping centers as ready-to-eat products, and various advantages, such as the fact that they are healthy and fresh products.
- microbial quality
- sensory quality
- edible coating and films
- natural antioxidants
1. Introduction
In recent years, fruit consumption has increased in a large portion of the population due to the current concern for a healthy lifestyle [1]. Fruits are a rich source of vitamins, minerals, and dietary fiber, which are essential for the human diet. Due to a population that is very busy with their different activities, and which consequently has less time to prepare its food, there has been a tendency to demand fresh, nutritious, convenient, and quickly accessible products, such as fresh-cut produce. Therefore, fresh-cut fruits constitute one of the fastest-growing food industry segments [2]. The Food and Drug Administration (FDA) defines fresh-cut fruit as any fruit that has been changed physically from its natural state by minimal processing, such as chopping, dicing, peeling, shredding, slicing, spiralizing, or tearing [3]. Fresh-cut fruits remain fresh without additional treatment, such as blanching, freezing, canning, cooking, or adding juice, syrup, or dressing. These products can be considered ready-to-eat but may or may not be washed before being packed for use by the customer or a retail food store. Fresh-cut fruits can include single or mixed fruits in the same packaging, providing great convenience, nutritional value, taste, and freshness [3].
The maintenance of the quality of fresh-cut fruits is attributed to the physiological and biological mechanisms of each fruit and environmental conditions such as storage conditions, temperature, and humidity [4]. The main disadvantage of fresh-cut fruits is their short shelf-lives, often less than two weeks [5]. When fresh fruits are cut or minimally processed, they are susceptible to chemical, physical, microbiological, and sensory changes. This affects the product’s marketability and reduces its nutritional value, and several foodborne outbreaks are linked to fresh-cut produce [1]. Additionally, it must be considered that many other factors can impact the final product, such as the type of packaging, postharvest processing, and type of cultivar, among others [6]. Whole fruits have a natural barrier (peel) that protects them from spoilage, microorganisms, or environmental conditions; however, this barrier is typically removed during the processing of fresh-cut fruits, making them more susceptible to decay (Figure 1) [7]. It is even more challenging to maintain the quality of fresh-cut fruits than vegetables due to their complicated physiology [8].
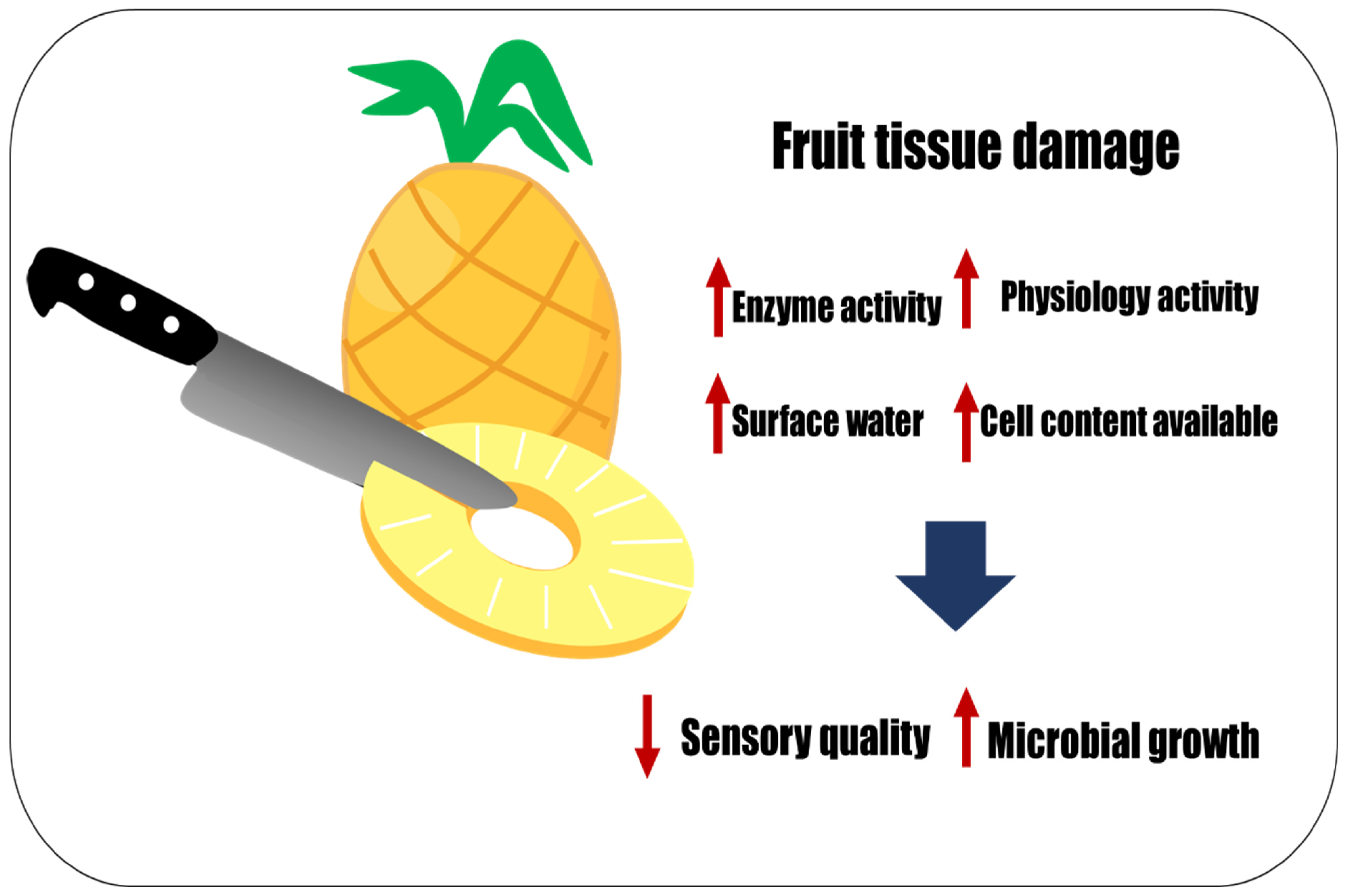
Figure 1. Minimal processing affects sensory quality and promotes microbial growth. The red up arrow indicates increase and the red down arrow indicates decrease.
Several methods could be employed to preserve fruits; however, as fresh-cut fruits are consumed fresh, thermal or freeze techniques are unsuitable because they impair the sensory, nutritional, and physicochemical quality. As a result, finding effective strategies to prevent microorganism growth while maintaining the quality of fresh-cut fruits is of great interest to food industries. The conventional way of retaining the fresh-cut products’ microbiological quality is through washing and disinfection with chlorine due to its low cost and ease of use. However, it has been hypothesized that chlorination’s disinfection byproducts may pose a health risk to humans [9]. Moreover, consumers demand more natural and fresh products without synthetic additives, making them nutritionally healthier. Therefore, technologies such as active packing, natural additives, ultraviolet light, high hydrostatic pressure, and ozone treatments are emerging to improve fresh-cut fruits’ microbiology and sensory quality.
2. Fresh-Cut Fruit Processing Impacts Physicochemical, Sensory, and Microbial Quality
When fresh fruits are cut or minimally processed (peeling, cutting, or slicing), they are susceptible to chemical, physical, sensory, and microbiological changes. Wounding during processing results in an increase in off-flavor compounds, loss of firmness and respiration, reduced fresh-cut shelf life, and senescence processes [4]. One of the degradation factors of these products is the color change. For the consumer, the product’s first appearance is significant, since each product’s characteristic color indicates its freshness and quality [10]. Polyphenol oxidase (PPO) and peroxidase (POD) are the main enzymes that cause color degradation and degradation of other sensory properties, such as taste and flavor, in fresh-cut fruits [11]. In particular, the PPO enzyme catalyzes the oxidation of polyphenols to o-quinones in the presence of oxygen, producing undesirable pigments (brown coloration of many fruits and vegetables) during ripening, storage, processing, and handling. At the same time, the POD enzyme (considered antioxidant) catalyzes the conversion of hydrogen peroxide to water using various substrates, such as polyphenols, lipids, or other compounds [12]. There are several studies focused on the inhibition of these enzymes using different inhibitory compounds, such as volatile compounds, cysteine, and ascorbic acid [13][14]; or by other processes, such as microwaving [15], ultrasonication [16], high isostatic pressure, and thermal conditions [17][18].
Flavor and texture are other attributes that must be considered in consumer satisfaction. The flavor is commonly related to aroma (odor) and taste (salty, sour, bitter, sweet, and umami). Umami is described by salts of amino acids and nucleotides [19]. The flavor in fresh-cut fruits can be affected by the increases in sugar contents, decrease in organic acids, and changes in aroma (volatile compounds) [10]. Enzymes such as lipoxygenase or peroxidase lead to the development of undesirable flavors—rancid, cardboard, or oxidized off-flavors (Figure 2) [20].
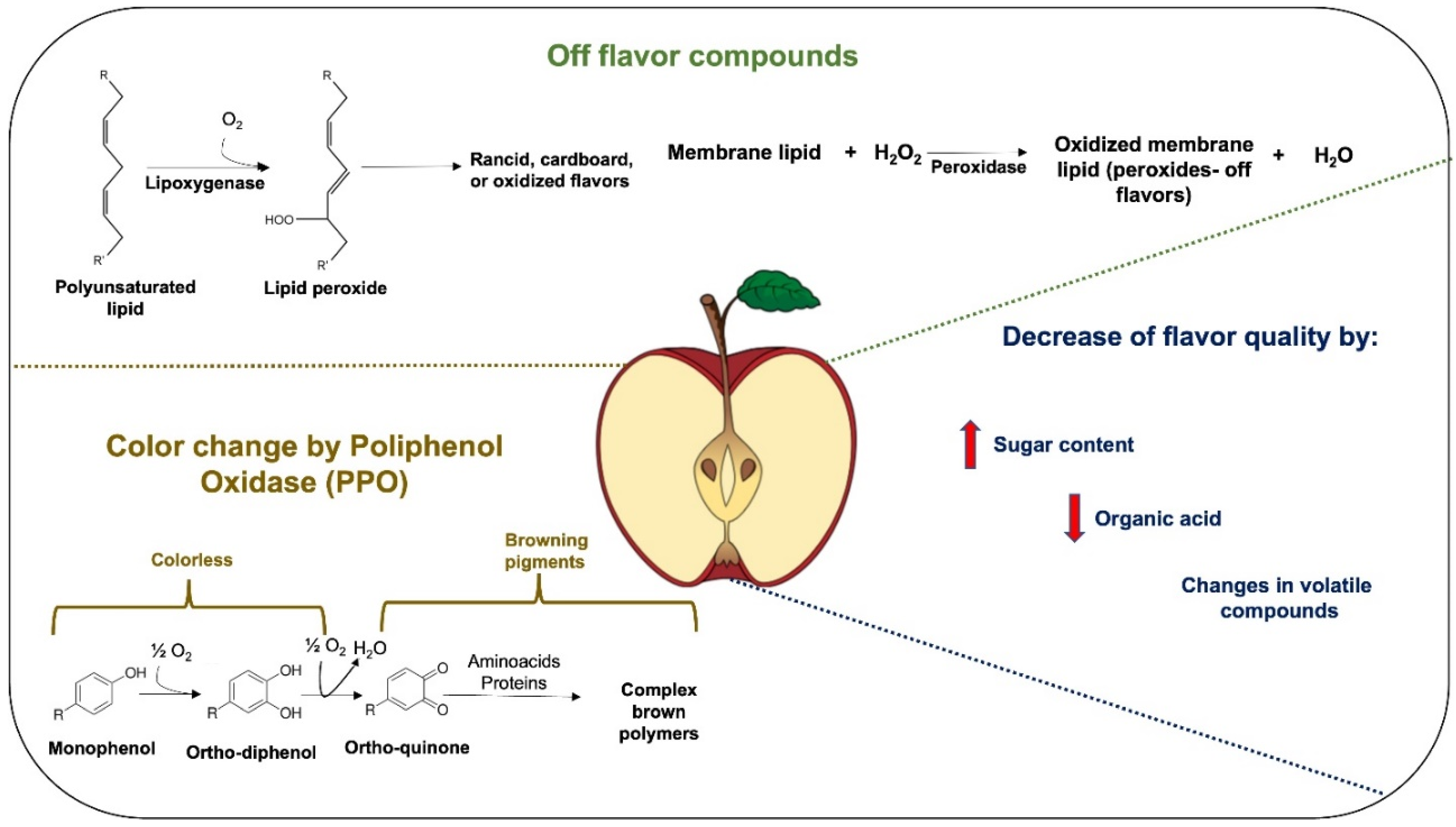
Figure 2. Enzymatic reactions and other factors that produce browning and off-flavors of fresh-cut fruits. The red up arrow indicates increase and the red down arrow indicates decrease.
On the other hand, the textural properties of fresh-cut fruits are related to the deformation, disintegration, and flow of the food under a force. The texture is related to the cell wall components (pectin, hemicellulose, and cellulose), generating the softening of fresh-cut fruits during storage by enzymatic or non-enzymatic mechanisms. This is due to the loss of cohesiveness, which decreases intermolecular bonding between cell wall polymers. Consequently, an increase in the solubility of the wall constituents is observed, principally pectin, generating the softening. Other factors, such as pH, salt concentration, and water, deficiency could influence texture loss [21].
Salinas-Hernández et al. [22] predicted fresh-cut mango’s shelf life, indicating that physicochemical variables related to sensory changes can be identified and used as quality indicators. Therefore, physicochemical variables (weight, volume, pH, titratable acidity, soluble solids content, volatiles) with high correlations with the sensory attributes (color, texture, and flavor) can be used as indicators of the sensory changes, since they are easy to measure, objective, and relatively inexpensive. Additionally, the identification of these variables was carried out by regression analysis. There are several studies relating physicochemical and sensory characteristics of papaya [23], persimmon [24], plums [25], mango-based fruit bar [26], jackfruit [27], apples [28], and oranges [29], among other fresh-cut fruits.
Fresh-cut fruits are considered perishable due to their intrinsic characteristics and minimal processing that favor microbial growth, which can cause changes in safety and sensory and physicochemical properties. Fresh-cut fruit cells naturally deteriorate with time and are influenced by conditions after harvest, processing, and storage. Peeling and processing cause physiological damage and cell disruption, resulting in the release of their components, such as carbohydrates and proteins, which serve as a source of nutrients for native or exogenous microorganisms [30]. Minimal processing destroys living plants’ protective membranes and barriers, allowing microbial pathogens to enter and contaminate them. The chances of food-borne illness due to pathogens or spoilage organisms growing in these products are very high. In addition, fresh-cut fruits have water activity levels between 0.8 and 0.99 and pH values between 3.0 and 7.5, suitable conditions for the growth of microorganisms [2]. The types and growth rates of the microorganisms will be significantly influenced by the product’s temperature, the relative humidity, the atmosphere, and intrinsic factors such as pH, water content, and nutrients [31].
Fresh-cut fruits are prone to the invasion and colonization of microorganisms such as mesophiles and psychrophiles, molds, and yeasts [2]. Two types of microorganisms can contaminate these products: deteriorative and pathogenic. Deteriorative microorganisms cause damage to fresh-cut fruits and make them sensorily unacceptable. For example, this type of microorganism causes the production of lactic acid, acetic acid, hydrogen gases, and carbon dioxide, which results in sour odors and puffing up of packages. Other products include thiols, esters, amines, and peroxides that cause off-flavors, odors, and color changes. In addition, spoilage microorganisms can produce enzymes such as proteases, lipases, and amylases that cause structural changes in tissue and flavor [31]. The incidence of pathogenic microorganisms represents a food safety risk. Fresh-cut fruits when consumed raw are vehicles for the transmission of pathogens. Among the most common pathogenic microorganisms found in fresh-cut fruits are bacteria such as Listeria monocytogenes, Escherichia coli, Salmonella enterica, Campylobacter spp., and Staphylococcus aureus; fungi such as Alternaria spp., Penicillium spp., Botrytis spp., Rhizopus spp., and Colletotrichum spp.; and some viruses, such as norovirus and hepatitis A [2][32][33][34]. These microorganisms are responsible for many outbreaks worldwide [34]. The microbiological safety of fresh-cut fruits is crucial to maintaining their commercial value. Microbial contamination has an economic impact on food loss because it reduces the product’s shelf life through deterioration and risks the public’s health by causing foodborne illnesses [30][35]. As these products are consumed without any thermal treatment, it is essential to keep the microbial loads of fresh-cut fruits as low as possible to avoid foodborne diseases.
One of the key elements influencing the quality of fresh-cut fruits is exposure to cold temperatures immediately after harvest to reduce the effects of cutting stress. Sometimes a temperature just above that which would cause chilling injury gives the optimal condition for quality. Due to the perishable nature of fresh-cut fruits, on some occasions, it is preferable to store them under refrigeration at a temperature that could cause slight chilling injury as opposed to one that would promote quick natural deterioration [36]. Chilling damage to fresh-cut fruits can have various symptoms, some of which are noted despite minor visual manifestations; for example, poor flavor retention associated with the inhibition of volatile aroma production, increased respiration rates in fresh-cuts relative to the corresponding whole fruit, and tissue transparency and juice leakage because membrane damage, particularly in fresh-cut tropical and subtropical fruit. The signs of chilling injury in the entire fruit can also be generalized for fresh-cut products, such as softening or other textural alterations, pigment loss, and increased CO2 production [37]. In fresh-cut fruits, chilling injury symptoms are caused mainly by the physical shifting of the membrane from a liquid–crystalline to a solid–gel phase during chilling. This process is highly reliant on the degree of membrane lipid saturation. The fluidity-dependent membranes start solidifying at cool temperatures, causing membrane integrity/leakiness problems, solute diffusion, tissue water loss, and membrane-bound protein agglomeration. Due to disruptions in the membranes connected to the electron transport chain, suboptimal chilling temperatures may also hasten oxidatively induced senescence and increase the accumulation of active oxygen species [36].
Cold storage temperatures have an impact on final product quality. For example, fresh-cut mango, a tropical fruit, is susceptible to chilling injury when stored at low temperatures, compromising its overall sensory quality. The ideal storage temperature for fresh-cut fruits is never more than 5 °C, a chilling temperature for freezing delicate tropical fruits such as mangoes. Despite the possibility of chilling injury, Dea, Brecht, Nunes, and Baldwin [37] showed that fresh-cut mango slices had a longer shelf-life when stored at 5 °C than at 12 °C because the negative changes in appearance and aroma that occurred at the higher temperature were more unpleasant than the moderate negative changes that appeared at a lower temperature. Marrero and Kader [38] reported that temperature was the primary factor affecting the quality of fresh-cut pineapple. The pulp pieces’ post-cutting life was 4 days at 10 °C, yet more than 2 weeks at 0 °C. This increased longevity at levels below the chilling injury limit is also the case for whole fruits. At all temperatures, a rapid increase in respiration followed by an increase in ethylene production marked the end of commercial life. Beyond this point, storage was continued, which resulted in the development of off-tastes, smells, and microbiological deterioration. Even after being maintained at 0 °C for two weeks, the pulp fragments exhibited no symptoms of chilling damage. Since some of the symptoms may not appear for several days after being transferred to non-chilling temperatures, this may be because of the short time (approximately 3 h) between removal from refrigerated storage and quality evaluation. The impacts of temperature on fresh-cut fruits’ physicochemical, sensory, and microbiological quality still require more research.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae8080731
References
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830.
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748.
- FDA. Draft Guidance for Industry: Guide to Minimize Food Safety Hazards of Fresh-Cut Produce. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-guide-minimize-food-safety-hazards-fresh-cut-produce#:~:text=In%20this%20guidance%2C%20%E2%80%9Cfresh%2D,without%20additional%20processing%20(such%20as (accessed on 12 May 2022).
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209.
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of cultivar, anti-browning solutions, packaging gasses, and advanced technology on browning in fresh-cut apples during storage. J. Food Process Eng. 2017, 40, e12400.
- Siddiq, M.; Harte, J.; Beaudry, R.; Dolan, K.; Singh, S.; Saha, K. Physicochemical properties of whole fruit and sensory quality of fresh-cut apples pre-treated with 1-Methylcyclopropene (1-MCP). Int. J. Food Prop. 2014, 17, 1081–1092.
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Soliva-Fortuny, R.; Martín-Belloso, O. Control of pathogenic and spoilage microorganisms in fresh-cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180.
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.A.; Kovačević, D.B. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419.
- Weisman, R.J.; Heinrich, A.; Letkiewicz, F.; Messner, M.; Studer, K.; Wang, L.; Regli, S. Estimating national exposures and potential bladder cancer cases associated with chlorination DBPs in US drinking water. Environ. Health Perspect. 2022, 130, 087002.
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chem. X 2020, 8, 100107.
- Wen, Y.T.; Liang, Y.Q.; Chai, W.M.; Wei, Q.M.; Yu, Z.Y.; Wang, L.J. Effect of ascorbic acid on tyrosinase and its anti-browning activity in fresh-cut Fuji apple. J. Food Biochem. 2021, 45, e13995.
- Lee, J.H.; Kasote, D.M.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Effect of production system and inhibitory potential of aroma volatiles on polyphenol oxidase and peroxidase activity in tomatoes. J. Sci. Food Agric. 2021, 101, 307–314.
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439.
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by L-cysteine. Food Chem. 2018, 266, 1–8.
- De Barcelos Costa, H.C.; Siguemoto, É.S.; Cavalcante, T.A.B.B.; de Oliveira Silva, D.; Vieira, L.G.M.; Gut, J.A.W. Effect of microwave-assisted processing on polyphenol oxidase and peroxidase inactivation kinetics of açai-berry (Euterpe oleracea) pulp. Food Chem. 2021, 341, 128287.
- Ji, D.; Wang, Q.; Lu, T.; Ma, H.; Chen, X. The effects of ultrasonication on the phytochemicals, antioxidant, and polyphenol oxidase and peroxidase activities in coffee leaves. Food Chem. 2022, 373, 131480.
- De Jesus, A.L.T.; Leite, T.S.; Cristianini, M. High isostatic pressure and thermal processing of açaí fruit (Euterpe oleracea Martius): Effect on pulp color and inactivation of peroxidase and polyphenol oxidase. Food Res. Int. 2018, 105, 853–862.
- Engmann, F.N.; Ma, Y.; Zhang, H.; Yu, L.; Deng, N. The application of response surface methodology in studying the effect of heat and high hydrostatic pressure on anthocyanins, polyphenol oxidase, and peroxidase of mulberry (Morus nigra) juice. J. Sci. Food Agric. 2014, 94, 2345–2356.
- Istiqamah, A.; Lioe, H.N.; Adawiyah, D.R. Umami compounds present in low molecular umami fractions of asam sunti–A fermented fruit of Averrhoa bilimbi L. Food Chem. 2019, 270, 338–343.
- Meng, K.; Hou, Y.; Han, Y.; Ban, Q.; He, Y.; Suo, J.; Rao, J. Exploring the functions of 9-lipoxygenase (DkLOX3) in ultrastructural changes and hormonal stress response during persimmon fruit storage. Int. J. Mol. Sci. 2017, 18, 589.
- Defilippi, B.G.; Ejsmentewicz, T.; Covarrubias, M.P.; Gudenschwager, O.; Campos-Vargas, R. Changes in cell wall pectins and their relation to postharvest mesocarp softening of “Hass” avocados (Persea americana Mill.). Plant Physiol. Biochem. 2018, 128, 142–151.
- Salinas-Hernández, R.M.; González-Aguilar, G.A.; Tiznado-Hernández, M.E. Utilization of physicochemical variables developed from changes in sensory attributes and consumer acceptability to predict the shelf life of fresh-cut mango fruit. J. Food Sci. Technol. 2015, 52, 63–77.
- Jayathunge, K.; Gunawardhana, D.; Illeperuma, D.; Chandrajith, U.; Thilakarathne, B.; Fernando, M.; Palipane, K. Physico-chemical and sensory quality of fresh cut papaya (Carica papaya) packaged in micro-perforated polyvinyl chloride containers. J. Food Sci. Technol. 2014, 51, 3918–3925.
- Sanchís, E.; Mateos, M.; Pérez-Gago, M.B. Physicochemical, sensory, and nutritional quality of fresh-cut “Rojo Brillante” persimmon affected by maturity stage and antibrowning agents. Food Sci. Technol. Int. 2016, 22, 574–586.
- Kitzberger, C.S.G.; da Silva, C.M.; dos Santos Scholz, M.B.; Ferreira, M.I.F.; Bauchrowitz, I.M.; Eilert, J.B.; dos Santos Neto, J. Physicochemical and sensory characteristics of plums accesses (Prunus salicina). AIMS AgriFood 2017, 2, 101–112.
- Leguizamon-Delgado, M.A.; Duque-Cifuentes, A.L.; Quintero-Castaño, V.D. Physico-chemical and sensory evaluation of a mango-based fruit bar. Dyna 2019, 86, 276–283.
- Rana, S.S.; Pradhan, R.C.; Mishra, S. Image analysis to quantify the browning in fresh cut tender jackfruit slices. Food Chem. 2019, 278, 185–189.
- Zhao, Q.; Tang, S.; Fang, X.; Wang, Z.; Jiang, Y.; Guo, X.; Zhu, J.; Zhang, Y. The Effect of Lactiplantibacillus plantarum BX62 alone or in combination with chitosan on the qualitative characteristics of fresh-cut apples during cold storage. Microorganisms 2021, 9, 2404.
- Glicerina, V.; Siroli, L.; Betoret, E.; Canali, G.; Dalla Rosa, M.; Lanciotti, R.; Romani, S. Characterization and evaluation of the influence of an alginate, cocoa and a bilayer alginate–cocoa coating on the quality of fresh-cut oranges during storage. J. Sci. Food Agric. 2022, 102, 4454–4461.
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-cut fruits and vegetables: Quality issues and safety concerns. In Fresh-Cut Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15.
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial spoilage of fruits: A review on causes and prevention methods. Food Rev. Int. 2020, 1–22.
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 335, 108855.
- Ortiz-Solà, J.; Valero, A.; Abadias, M.; Nicolau-Lapeña, I.; Viñas, I. Evaluation of water-assisted UV-C light and its additive effect with peracetic acid for the inactivation of Listeria monocytogenes, Salmonella enterica and murine norovirus on whole and fresh-cut strawberries during shelf-life. J. Sci. Food Agric. 2022, 1–10.
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667.
- Hasan, S.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2020, 55, 1–10.
- Hodges, D.M.; Toivonen, P.M. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 155–162.
- Dea, S.; Brecht, J.K.; Nunes, M.C.N.; Baldwin, E.A. Occurrence of chilling injury in fresh-cut ‘Kent’mangoes. Postharvest Biol. Technol. 2010, 57, 61–71.
- Marrero, A.; Kader, A.A. Optimal temperature and modified atmosphere for keeping quality of fresh-cut pineapples. Postharvest Biol. Technol. 2006, 39, 163–168.
This entry is offline, you can click here to edit this entry!
