Thermo-electrochemical cells (thermocells; TECs) represent a promising technology for harvesting and exploiting low-grade waste heat (<100–150 °C) ubiquitous in the modern environment. Based on temperature-dependent redox reactions and ion diffusion, emerging liquid-state thermocells convert waste heat energy into electrical energy, generating power at low costs, with minimal material consumption and negligible carbon footprint. New thermoelectric applications in the field include wearable and portable electronic devices in the health and performance monitoring sectors, using body heat as a continuous source of energy etc. For waste heat conversion to partially replace fossil fuels as an alternative energy resource, power generation needs to be commercially viable, cost effective and sustainable.
- Thermo-electrochemical cells
- energy harvesters
- low grade waste heat
- wearable electronics
- micro supercapacitors
- thermoelectrics
[1]1. Introduction
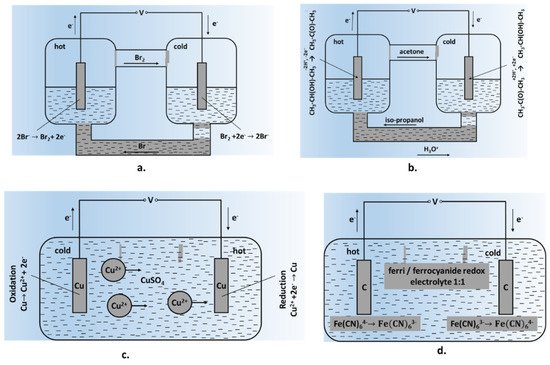
2. Redox Couples and Electrolytes
2.1. Redox Couples
2.2. Electrolytes
3. Electrode Materials and Designs
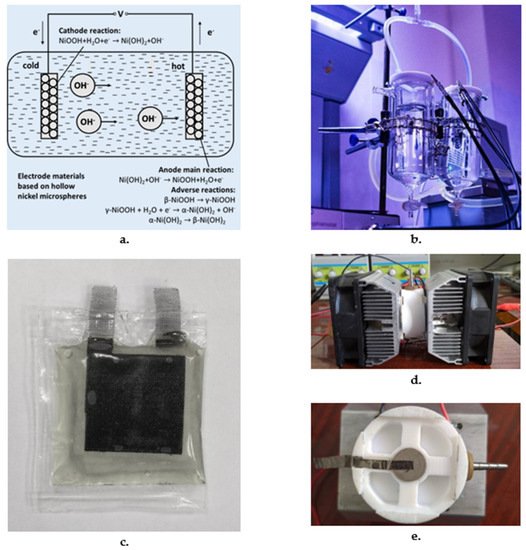
Additional electrode designs: The flexible nature of nano-carbons has led to new thermocell designs suitable for wrapping around curved or irregularly shaped surfaces such as vehicle exhaust pipes, cooling/heating lines in power plants. Hu et al. [81] used two layers of Nomex HT 4848 to separate two MWNT bucky paper electrodes at a distance of 2mm; these scrolled electrodes increased the relative efficiency of the cell threefold to 1.4% relative to a Carnot engine. Thin coin like thermocells have been developed using chemical vapor deposition by producing a catalyst with layers of Ti (30 nm), Al (10 nm) and Fe (2nm) and using it to grow 100µm tall MWNT forests on the thermocell casing. Nomex HT 4848 separators impregnated with 0.4 M ferri/ferro cyanide solution kept the electrodes apart; an aerial power density of 0.98 W/m2 was obtained for DT of 60ºC [30]. Stacked electrode configuration using SWNT and rGO (reduced graphene oxide) (in 9:1 proportion) consisting of layers of stainless-steel mesh as separators between 4.5µm electrode films maintaining a conductive path between individual films. A 10-stack configuration attained an efficiency of 2.63 % relative to a Carnot engine. Marquardt et al. [85] have reported on a thermocell based on a proton exchange membrane with hydrogen electrodes; maximum open circuit voltage with a power density of 45:3 mW/cm2 was observed at humidifier temperature of 323K. A Seebeck coefficient of 1.75 mV/K was observed for DT of 35ºC.
- Emerging applications
The conversion of waste heat to electrical energy is finding application in several fields. Some of the emerging applications are presented in this section. While some of these are stand alone on TE effect, others are in conjunction with other technologies.
Wearable and portable devices: Wearable electronic devices are gaining attention in the health and performance monitoring sectors towards long-term, continuous, self-powered operations using human body as a continuous supply of energy. Harvesting of body heat in wearable devices has been investigated extensively as an alternative to bulky batteries that require frequent charging or replacement [86,87] . As the core body temperature is regulated at 37°C, body heat can be a continuous source of energy; the total heat dissipated from the human body can range between 60 to 180W depending on the activity level [88]. As only a small fraction of body surface can be covered by TEG devices, overall efficiencies are likely to be quite small. Of the several approaches used for harvesting body heat such as electrostatic, piezoelectric, electromagnetic, pyroelectric and thermoelectric effects, we will focus our attention on thermoelectric mechanisms [89]. Wearable electronic devices find application in medical devices (real time monitoring, blood pressure sensors, ear-ware), smart watches, sportswear, wristbands, flexible devices for monitoring non-flat surfaces in industrial applications [90].
Among the well-known thermoelectric materials, alloys of bismuth telluride with antimony and selenium have been investigated extensively for room temperature operations. (BixSb1-x)Te3 alloy system is a p- type semiconductor legs with ZT ranging between 1-2; Bi2Te3-xSe x is an n-type semiconductor legs with ZT around 1 [91–93]. An array of p- and n-type semiconductor legs are arranged in a thermoelectric module and are electrically connected in series, and then thermally connected in parallel towards greater overall efficiency [94]. A schematic representation of a typical TEG circuit in the form of a wrist band is shown in Fig. 3 for harvesting body heat.
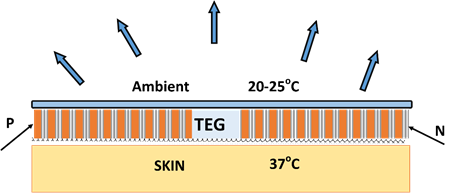
Fig. 3. A schematic representation of a TEG wrist band for harvesting body heat
In rigid TEGs, n- and p-legs and interconnects are typically affixed to a thermally conductive ceramic substrate (alumina or aluminum nitride). In flexible TEGs, Gallium-Indium eutectic alloys have been used as flexible liquid-metal interconnects to ascertain integrity during operation; Kapton HN and polydimethylsiloxane are the two most common substrates [95]. Typical power outputs are found to range between 2 to 8.75µW/cm2 [96]. Liu et al [97] have presented several different designs for wearable devices for harvesting body heat in a range of applications.
Organic materials have also been investigated as flexible TEGs due to lower prices, light weight, low thermal conductivity, and high flexibility. While significant progress has been made, high performance organic TEGs still lag behind inorganic chalcogenides BiTe-based alloys. One of the strategies is to fabricate flexible TE devices from composites of organic and polymeric materials with micro/nanomaterials [98]. The TE properties of several p-type and n-type coordination polymers have been tabulated by Masoumi et al. [98]. π conjugated conducting polymers, are another group of organic TEs, with a capacity for doping with a wide variety of elements and adjustable doping levels [95]. Some of key conducting polymers include polyacetylene, polyvinylidene fluoride, polyphenylenevinylene, poly(3-hexylthiophene), polypyrrole etc. A power factor of 401 µW/ mK2 and a Seebeck coefficient of 43.5 µV/K has been achieved with some conducting polymers [99]. Other TE materials include carbon nanotubes, graphene, fullerenes, carbon nanodots, small molecules, organic composites with inorganic fillers etc. [94].
Energy storage devices: The integration of energy-harvesting and storage devices has been extensively investigated for emerging self-powered electronic devices [97]. Thermoelectric generators can be used to convert excess heat generated during the operation of electronic devices into electricity [93]. Using Soret effect, ionic thermoelectric supercapacitors utilize ionic electrolytes to produce charges and energy storage in a single device; the operation of these devices is however limited by long charging and discharging times and a rigid configuration [100]. Yang et al. [101] have reported on a TEG device with n- and p- type modules consisting of Ag2Te and Ag2Se nanoparticle thin films. This device was directly linked with a planar micro supercapacitor. A Seebeck voltage of 82 mV was generated for DT of 15.8 K and a charging efficiency of 98%. Wu et al. [102] have reported on novel conjugated conducting polymer (PDAQ-BC) [DAQ: 2,6-diaminoanthraquinone; DBC: 3,6-dibromo-9-(4-bromophenyl)carbazole], which showed a specific capacitance of 180.5 F/g for a current of 1A/g. This polymer retained up to 95% coulombic efficiency and 85% capacitance after 5000 cycles of operation.
Park et al. [103] have reported an all-in-one energy system consisting of a TEG on one side of the substrate and a micro supercapacitor (MSC) on the other side. The TEG was constructed from screen printed p- and n- modules and a p- type TE film for alignment with electrodes. An MSC was fabricated on the other side using rGO/CNT electrodes and Au current collectors. Electrodes were positioned above and below the TEG-substrate-MSC system; the electrode on the TEG side was connected externally to the current collector of MSC. This system was able to generate 10.8V of electrical energy for thermal differences up to 10K and store it without loss.
Liu et al. [107] have reported on coupling thermoelectricity and electrocatalysis for hydrogen production via PbTe-PbS/TiO2 heterojunction involving a cathode, an anode and electrolyte operating at the hot-end and in-situ endothermic production of electrochemical hydrogen on the cold-end. At 70ºC and 1.0V bath voltage, hydrogen was generated at the rate of 6.1mL/cm2/h with energy and heat efficiencies reaching 88% and 49.9% respectively. Liu et al. [108] have reported on an advanced Li ion battery with charging based on a coupled thermoelectric approach. Richards et al. [109] have explored solid-state electrochemical heat engines (EHE) for generating electric power from available thermal energy based on reversible redox reactions. EHEs control and utilize the electrochemical potential of molecules undergoing redox reactions with temperature, composition and pressure playing an important role.
Liao et al. [110] have reported on a hybrid active/passive battery thermal management system in combination with thermoelectric elements (TEE) and phase change materials for the management of Li-ion batteries operating in extreme environments. TEEs were used to provide refrigeration at high temperatures and heating to preheat the batteries in cold environments. Nguyen and Shabani [111] have discussed about capturing the heat produced by proton exchange membrane fuel cells, heat recovery solutions and opportunities for integration with TEGs and thermally regenerative electrochemical cycles for power cogeneration applications.
- Conclusions and future perspectives
Development of thermoelectrochemical technologies for harvesting waste low-temperature heat opens the prospect of increasing the efficiency of various devices and mechanisms operating in exothermic mode or creating systems for generating electricity based on natural heat sources. Any process that can partially replace fossil fuels as a prime energy source will be used only if it is attractive to industry; an alternative energy source such as waste heat conversion (WHC) has to cost effective to becoming commercially viable. Geoffroy et al. [112] have shown that current WHC heat engines are not economically viable below 100ºC and require temperatures above 150ºC coupled with 100-1000 kW power outputs to be economically competitive. Studies in recent years have shown the possibility of significant increases in the power and conversion efficiency of TEC cells. Highest values of output power and cell potentials have been achieved for the redox ferri/ferrocyanide system and Co2+/3+, which offers great opportunities for further development and research in both aqueous and non-aqueous solvents.
Achieved results show the pathways to overcome key fundamental limitations of thermocell performance and set new tasks for fundamental research and further development of electrodes, electrolyte materials and cell design. One of the key tasks in thermocells development is to investigate mechanisms of entropy change via new redox couples and electrolytes. This will be related to increasing the hypothetical Seebeck coefficient, as well as improving the properties of electrodes and solvents to increase the mass transfer rate (diffusion capacity) towards increasing exchange currents and output power values.
One of the most promising thermoelectric power generation application involves vehicle waste heat recovery to improve fuel economy, wherein waste heat from the exhaust, is redirected to produce electricity. Other applications include harvesting industrial waste heat (incinerators, cement, steel mills etc.), geothermal, fuel oil-fired furnaces or gas water-heaters with this technology. Despite extensive research, WHC technology has yet to achieve significant market penetration. For WHCs to become be a serious contender, it has to compete with solar, wind, geothermal technologies in terms of capacity factors, capital costs, operational as well as maintenance costs. Significant developments are therefore required on multiple fronts towards achieving greater power density performances especially at higher temperatures. It is possible that TECs might have major advantages over solar cells and semiconductor thermoelectrics, in particular in the Wh/dollar efficiency [81]. Achieving industrial production volumes of MWCNTs and reducing their cost, according to the author, can make TEC a competitive device.
This entry is adapted from the peer-reviewed paper 10.3390/su14159483
References
- Forman, C.; Muritala, I. K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renewable and Sustainable Energy Reviews 2016, 57, 1568–1579.
- Straub, A. P.; Yip, N. Y.; Lin, S.; Lee, J.; Elimelech, M. Harvesting low-grade heat energy using thermo-osmotic vapour transport through nanoporous membranes. Nature Energy 2016, 1, 1–6.
- Yang, Y.; Lee, S. W.; Ghasemi, H.; Loomis, J.; Li, X.; Kraemer, D.; Zheng, G.; Cui, Y.; Chen, G. Charging-free electrochemical system for harvesting low-grade thermal energy. Proc Natl Acad Sci U S A 2014, 111, 17011–17016.
- Evans, S. CarbonBrief/Energy, 2017. Solar, wind and nuclear have ‘amazingly low’ carbon footprints. Available online: https://www.carbonbrief.org/solar-wind-nuclear-amazingly-low-carbon-footprints/#:~:text=Simon%20Evans,-08.12.2017%20%7C%205&text=Building%20solar%2C%20wind%20or%20nuclear,of%20electricity%20out%20to%202050. (accessed on 10 Jan. 2022).
- COP26 Outcomes, 2021. Available online: https://ukcop26.org/the-conference/cop26-outcomes. (accessed on 10 Jan. 2022).
- Bouty, E. Phénomènes thermo-électriques et électro-thermiques au contact d’un métal et d’un liquide. Journal de Physique Théorique et Appliquée 1880, 9, 306–320.
- Snyder, G. J.; Toberer, E. S. Complex thermoelectric materials. Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group 2010, 101–110.
- Jangonda, C.; Patil, K.; Kinikar, A.; Bhokare, R.; Gavali, M. D. Review of various application of thermoelectric module. Intl. J. Innovative Research in Science, Engineering and Technology 2016, 5, 3393.
- Yang, X.; Wang, C.; Lu, R.; Shen, Y.; Zhao, H.; Li, J.; Zheng, X; Progress in Measurement of Thermoelectric Properties of Micro/Nano Thermoelectric Materials: A Critical Review. Nano Energy 2022, 107553.
- Chen, X. Q.; Fan, S. J.; Han, C.; Wu, T.; Wang, L. J.; Jiang, W.; Yang, J. P. Multiscale architectures boosting thermoelectric performance of copper sulfide compound. Rare metals 2021, 40(8), 2017-2025.
- Chen, X.; Zhang, H.; Zhao, Y.; Liu, W. D.; Dai, W.; Wu, T.; Yang, J. Carbon-encapsulated copper sulfide leading to enhanced thermoelectric properties. ACS applied materials & interfaces 2019, 11(25), 22457-22463.
- Vining, C. B. An inconvenient truth about thermoelectrics. Nature Materials 2009, 8, 83–85.
- deBethune, A. J.; Licht, T. S.; Swendeman, N. The Temperature Coefficients of Electrode Potentials. Journal of The Electrochemical Society 1959, 106, 616.
- Landry, B. A. Utilization of waste heat. Science 1953, 3, 3.
- Wakao, N.; Nojo, K. Nitric acid cycle process for extracting thermal energy from low-level heat sources. Nature 1978, 273, 25–27.
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602.
- Waske, A.; Dzekan, D.; Sellschopp, K.; Berger, D.; Stork, A.; Nielsch, K.; Fähler, S. Energy harvesting near room temperature using a thermomagnetic generator with a pretzel-like magnetic flux topology. Nature Energy 2018, 4, 68–74.
- Li, T.; Zhang, X.; Lacey, S. D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nature Materials 2019, 18, 608–613.
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z. L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346.
- Pandya, S.; Wilbur, J.; Kim, J.; Gao, R.; Dasgupta, A.; Dames, C.; Martin, L. W. Pyroelectric energy conversion with large energy and power density in relaxor ferroelectric thin films. Nature Materials 2018, 17, 432–438.
- Thakre, A.; Kumar, A.; Song, H. C.; Jeong, D. Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, Vol. 19, Page 2170 2019, 19, 2170.
- Quoilin, S.; Broek, M. van den; Declaye, S.; Dewallef, P.; Lemort, V. Techno-economic survey of Organic Rankine Cycle (ORC) systems. Renewable and Sustainable Energy Reviews 2013, 22, 168–186.
- Zhang, X.; Chau, K.T. An automotive thermoelectric photovoltaic hybrid energy system using maximum power point tracking. Energy Convers Manag 2011, 52(1), 641e7.
- Zhang, H.; W Kong, W.; Dong, F.; Xu, H.; Chen, B.; Ni, M. Application of cascading thermoelectric generator and cooler for waste heat recovery from solid oxide fuel cells. Energy Convers Manag 2017, 148, 1382-1390.
- Li, P.; Cai, L.; Zhai, P.; Tang, X.; Zhang, Q.; Nino, M. Design of a concentration solar thermoelectric generator. J Electron Mater 2010, 39(9), 1522-1530.
- Dupont, M. F.; MacFarlane, D. R.; Pringle, J. M. Thermo-electrochemical cells for waste heat harvesting – progress and perspectives. Chemical Communications 2017, 53, 6288–6302.
- Chum, H. L.; Osteryoung, R. A. Review of thermally regenerative electrochemical cells. Solar Energy Research Institute 1981.
- Lalancette, J.-M.; Roussel, R. Metals intercalated in graphite. V. A concentration cell with intercalated bromine. Canadian Journal of Chemistry 1976, 54, 3541–3544.
- Endo, M.; Yamagishi, Y.; Inagaki, M. Thermocell with graphite fiber-bromine intercalation compounds. Synthetic Metals 1983, 7, 203–209.
- Inagaki, M.; Matsumoto, A.; Sakai, M.; Maeda, Y. A cell of carbon-fibers and nitric acid with temperature difference. Nippon Kagaku Kaishi 1983, 309–311.
- Bonetti, M.; Nakamae, S.; Roger, M.; Guenoun, P. Huge Seebeck coefficients in nonaqueous electrolytes. The Journal of Chemical Physics 2011, 134, 114513.
- Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E. B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renewable Energy 2020, 157, 1–8.
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J. S.; Ovalle-Robles, R.; Yang, H. D.; Kihm, K. D.; Baughman, R. H.; Lee, H. H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nature Communications 2016, 7.
- Artyukhov D.; Kiselev N.; Gorshkov N.; Kovyneva N.; Ganzha O.; Vikulova M.; Gorokhovsky A.; Offor P.; Boychenko E.; Burmistrov I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell Sustainability 2021, 13(3), 1377.
- Inagaki, M.; Itoh, E.; Maeda, Y. Durable Performance of Thermocell with Carbon Cloth and Nitric Acid. TANSO 1985, 1985, 134–136.
- Battistel, A.; Peljo, P. Recent trends in thermoelectrochemical cells and thermally regenerative batteries. Current Opinion in Electrochemistry 2021, 30, 100853.
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779.
- Disalvo, F. J. Thermoelectric cooling and power generation. Science (1979) 1999, 285, 703–706.
- Abraham, T. J.; Macfarlane, D. R.; Baughman, R. H.; Jin, L.; Li, N.; Pringle, J. M. Towards ionic liquid-based thermoelectrochemical cells for the harvesting of thermal energy. Electrochimica Acta 2013, 113, 87–93.
- Romano, M. S.; Razal, J. M.; Antiohos, D.; Wallace, G.; Chen, J. Nano-carbon electrodes for thermal energy harvesting. Journal of Nanoscience and Nanotechnology 2015, 15, 1–14.
- He, J.; Al-Masri, D.; MacFarlane, D. R.; Pringle, J. M. Temperature dependence of the electrode potential of a cobalt-based redox couple in ionic liquid electrolytes for thermal energy harvesting. Faraday Discussions 2016, 190, 205–218.
- Kang, T. J.; Fang, S.; Kozlov, M. E.; Haines, C. S.; Li, N.; Kim, Y. H.; Chen, Y.; Baughman, R. H. Electrical Power From Nanotube and Graphene Electrochemical Thermal Energy Harvesters. Advanced Functional Materials 2012, 22, 477–489.
- Zhang, L.; Kim, T.; Li, N.; June Kang, T.; Chen, J.; Pringle, J. M.; Zhang, M.; Kazim, A. H.; Fang, S.; Haines, C.; et al. High Power Density Electrochemical Thermocells for Inexpensively Harvesting Low-Grade Thermal Energy. Advanced Materials 2017, 29, 1605652.
- Maeda, Y.; Kitamura, H.; Itoh, E.; Inagaki, M. A new carbon fiber and nitric acid cell with a temperature difference between electrodes. Synthetic Metals 1983, 7, 211–217.
- Mua, Y.; Quickenden, T. I. Power Conversion Efficiency, Electrode Separation, and Overpotential in the Ferricyanide/Ferrocyanide Thermogalvanic Cell. Journal of The Electrochemical Society 1996, 143, 2558–2564.
- Hinterleitner, B.; Knapp, I.; Poneder, M.; Shi, Y.; Müller, H.; Eguchi, G.; Eisenmenger-Sittner, C.; Stöger-Pollach, M.; Kakefuda, Y.; Kawamoto, N.; et al. Thermoelectric performance of a metastable thin-film Heusler alloy. Nature 2019 576:7785 2019, 576, 85–90.
- Li, D.; Sun, R. R.; Qin, X. Y. Improving thermoelectric properties of p-type Bi2Te3-based alloys by spark plasma sintering. Progress in Natural Science: Materials International 2011, 21, 336–340.
- Russ, B.; Glaudell, A.; Urban, J. J.; Chabinyc, M. L.; Segalman, R. A. Organic thermoelectric materials for energy harvesting and temperature control. Nature Reviews Materials 2016, 1, 1–14.
- Li, M.; Hong, M.; Dargusch, M.; Zou, J.; Chen, Z. G. High-efficiency thermocells driven by thermo-electrochemical processes. Trends in Chemistry 2021, 3, 561–574.
- Cho, C.; Stevens, B.; Hsu, J.-H.; Bureau, R.; Hagen, D. A.; Regev, O.; Yu, C.; Grunlan, J. C.; Cho, C.; Stevens, B.; et al. Completely Organic Multilayer Thin Film with Thermoelectric Power Factor Rivaling Inorganic Tellurides. Advanced Materials 2015, 27, 2996–3001.
- Wang, H.; Hsu, J.-H.; Yi, S.-I.; Lae Kim, S.; Choi, K.; Yang, G.; Yu, C.; Wang, H.; Yi, S.; Kim, S. L.; et al. Thermally Driven Large N-Type Voltage Responses from Hybrids of Carbon Nanotubes and Poly(3,4-ethylenedioxythiophene) with Tetrakis(dimethylamino)ethylene. Advanced Materials 2015, 27, 6855–6861.
- Wang, W.; Shu, G.; Tian, H.; Huo, D.; Zhu, X. A bimetallic thermally-regenerative ammonia-based flow battery for low-grade waste heat recovery. Journal of Power Sources 2019, 424, 184–192.
- Cheng, C.; Dai, Y.; Yu, J.; Liu, C.; Wang, S.; Feng, S. P.; Ni, M. Review of Liquid-Based Systems to Recover Low-Grade Waste Heat for Electrical Energy Generation. Energy and Fuels 2021, 35, 161–175.
- Hu, R.; Xu, D.; Luo, X. Liquid Thermocells Enable Low-Grade Heat Harvesting. Matter 2020, 3, 1400–1402.
- Black, J. J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Physical Chemistry Chemical Physics 2016, 18, 20768–20777.
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Applied Energy Materials 2018, 1, 1424–1428.
- Burmistrov, I.; Artyukhov, D.; Shindrov, A.; Gorshkov N.; Gorokhovsky, A. Thermo-Electrochemical Cells for Low-Grade Waste Heat Conversion. In Nanotech Middle East 2017 Conference and Exhibition. Dubai; Dubai, 2017; pp. 4–6.
- Sahami, S.; Weaver, M. J. Entropic and enthalpic contributions to the solvent dependence of the thermodynamics of transition-metal redox couples: Part II. Couples containing ammine and ethylenediamine ligands. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 1981, 122, 171–181.
- Migita, T.; Tachikawa, N.; Katayama, Y.; Miura, T. Thermoelectromotive force of some redox couples in an amide-type room-temperature ionic liquid. Electrochemistry 2009, 77, 639–641.
- Laux, E.; Uhl, S.; Journot, T.; Brossard, J.; Jeandupeux, L.; Keppner, H. Aspects of Protonic Ionic Liquid as Electrolyte in Thermoelectric Generators. Journal of Electronic Materials 2016 45:7 2016, 45, 3383–3389.
- Anari, E. H. B.; Romano, M.; Teh, W. X.; Black, J. J.; Jiang, E.; Chen, J.; To, T. Q.; Panchompoo, J.; Aldous, L. Substituted ferrocenes and iodine as synergistic thermoelectrochemical heat harvesting redox couples in ionic liquids. Chemical communications 2016, 52, 745–748.
- Zinovyeva, V.; Nakamae, S.; Bonetti, M.; Roger, M. Enhanced Thermoelectric Power in Ionic Liquids. ChemElectroChem 2014, 1, 426–430.
- Kim, T.; Lee, J. S.; Lee, G.; Yoon, H.; Yoon, J.; Kang, T. J.; Kim, Y. H. High thermopower of ferri/ferrocyanide redox couple in organic-water solutions. Nano Energy 2017, 31, 160–167.
- Wang, H.; Zhao, D.; Ulla Khan, Z.; Puzinas, S.; Jonsson, M. P.; Berggren, M.; Crispin, X.; Wang, H.; Zhao, D.; Khan, Z. U.; et al. Ionic Thermoelectric Figure of Merit for Charging of Supercapacitors. Advanced Electronic Materials 2017, 3, 1700013.
- Krebs, S. Performance analysis of a Copper II Sulfate Pentahydrate based thermogalvanic cell. Electronic Theses and Dissertations 2015.
- Cabral, D. M.; Howlett, P. C.; MacFarlane, D. R. Electrochemistry of the tris(2,2‘-bipyridine) complex of iron(II) in ionic liquids and aprotic molecular solvents. Electrochimica Acta 2016, 220, 347–353.
- Yamato, Y.; Katayama, Y.; Miura, T. Effects of the Interaction between Ionic Liquids and Redox Couples on Their Reaction Entropies. Journal of The Electrochemical Society 2013, 160, H309–H314.
- Jiao, N.; Abraham, T. J.; MacFarlane, D. R.; Pringle, J. M. Ionic Liquid Electrolytes for Thermal Energy Harvesting Using a Cobalt Redox Couple. Journal of The Electrochemical Society 2014, 161, D3061–D3065.
- Salazar, P. F.; Stephens, S. T.; Kazim, A. H.; Pringle, J. M.; Cola, B. A. Enhanced thermo-electrochemical power using carbon nanotube additives in ionic liquid redox electrolytes. Journal of Materials Chemistry A 2014, 2, 20676–20682.
- Kazim, A. H.; Cola, B. A. Electrochemical Characterization of Carbon Nanotube and Poly (3,4-ethylenedioxythiophene)−Poly(styrenesulfonate) Composite Aqueous Electrolyte for Thermo-Electrochemical Cells. Journal of The Electrochemical Society 2016, 163, F867–F871.
- Wu, J.; Black, J. J.; Aldous, L. Thermoelectrochemistry using conventional and novel gelled electrolytes in heat-to-current thermocells. Electrochimica Acta 2017, 225, 482–492.
- Jin, L.; Greene, G. W.; MacFarlane, D. R.; Pringle, J. M. Redox-Active Quasi-Solid-State Electrolytes for Thermal Energy Harvesting. ACS Energy Letters 2016, 1, 654–658.
- Xiao, Y.; Zhong, X.; Guo, J.; Zhou, C.; Zuo, H.; Liu, Q.; Huang, Q.; Zhang, Q.; Diao, X. The role of interface between LiPON solid electrolyte and electrode in inorganic monolithic electrochromic devices. Electrochimica Acta 2018, 260, 254–263.
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable thermocells based on gel electrolytes for the utilization of body heat. Angewandte Chemie 2016, 128, 12229–12232.
- Quickenden, T. I.; Mua, Y. A Review of Power Generation in Aqueous Thermogalvanic Cells. Journal of The Electrochemical Society 1995, 142, 3985–3994.
- Gunawan, A.; Li, H.; Lin, C. H.; Buttry, D. A.; Mujica, V.; Taylor, R. A.; Prasher, R. S.; Phelan, P. E. The amplifying effect of natural convection on power generation of thermogalvanic cells. International Journal of Heat and Mass Transfer 2014, 78, 423–434.
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renewable Energy Focus 2019, 29, 42–48.
- Koo, M. H.; Yoon, H. H. Fabrication of carbon nanotubes and charge transfer complex-based electrodes for a glucose/oxygen biofuel cell. Journal of Nanoscience and Nanotechnology 2013, 13, 7434–7438.
- Nugent, J. M.; Santhanam, K. S. V.; Rubio, A.; Ajayan, P. M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Letters 2001, 1, 87–91.
- Hu, R.; A. Cola, B.; Haram, N.; N. Barisci, J.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Letters 2010, 10, 838–846.
- Kiselev, N.; Artyukhov, D.; Boychenko, E.; Gorshkov, N.; Glubokaya, A.; Burmistrov, I. Electrolyte concentration dependences of NiO based thermoelectrochemical cells performance. AIP Conference Proceedings 2022, 2456, 020005.
- Taganova, A. A.; Boychenko, E. A.; Kiselev, N. v.; Khaidarov, B. B.; Kolesnikov, E. A.; Yudin, A. G.; Vikulova, M. A.; Gorshkov, N. v.; Kuznetsov, D. v.; Burmistrov, I. N. Synthesis and Study of the Composition of Hollow Microspheres of NiO and NiO/Ni Composition for Thermoelectrochemical Energy Converters of Low-Potential Temperature Gradients of Thermal Units Into Electricity. Refractories and Industrial Ceramics 2021, 61, 715–719.
- Marquardt, T.; Kube, J.; Radici, P.; Kabelac, S. Experimental investigation of a thermocell with proton exchange membrane and hydrogen electrodes. International Journal of Hydrogen Energy 2020, 45, 12680–12690.
- Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.; Lach, J.; Lee, B.; Muth, J.; Oralkan, O.; Ozturk, M.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proceedings of the IEEE 2015, 103, 665–681.
- Ando Junior, O. H.; Maran, A. L. O.; Henao, N. C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renewable and Sustainable Energy Reviews 2018, 91, 376–393.
- Riemer, R.; Shapiro, A. Biomechanical energy harvesting from human motion: Theory, state of the art, design guidelines, and future directions. Journal of NeuroEngineering and Rehabilitation 2011, 8, 1–13.
- Invernizzi, F.; Dulio, S.; Patrini, M.; Guizzetti, G.; Mustarelli, P. Energy harvesting from human motion: materials and techniques. Chemical Society Reviews 2016, 45, 5455–5473.
- Llamas R. IDC Media Center. Worldwide Wearables Market to Nearly Double by 2021, According to IDC Internet Available online: https://www.idc.com/getdoc.jsp?containerId=prUS42818517 (accessed 22.11. 2021).
- Xu, Z.; Wu, H.; Zhu, T.; Fu, C.; Liu, X.; Hu, L.; He, J.; He, J.; Zhao, X. Attaining high mid-temperature performance in (Bi,Sb)2Te3 thermoelectric materials via synergistic optimization. NPG Asia Materials 2016 8:9 2016, 8, e302–e302.
- Nozariasbmarz, A. In-situ sintering decrystallization of thermoelectric materials using microwave radiation; North Carolina State University, 2017.
- Wang, S.; Tan, G.; Xie, W.; Zheng, G.; Li, H.; Yang, J.; Tang, X. Enhanced thermoelectric properties of Bi2(Te1−xSex)3-based compounds as n-type legs for low-temperature power generation. Journal of Materials Chemistry 2012, 22, 20943–20951.
- Leonov, V. Thermoelectric energy harvester on the heated human machine*. Journal of Micromechanics and Microengineering 2011, 21, 125013.
- Suarez, F.; Parekh, D. P.; Ladd, C.; Vashaee, D.; Dickey, M. D.; Öztürk, M. C. Flexible thermoelectric generator using bulk legs and liquid metal interconnects for wearable electronics. Applied Energy 2017, 202, 736–745.
- Park, H.; Lee, D.; Kim, D.; Cho, H.; Eom, Y.; Hwang, J.; Kim, H.; Kim, J.; Han, S.; Kim, W. High power output from body heat harvesting based on flexible thermoelectric system with low thermal contact resistance. Journal of Physics D: Applied Physics 2018, 51, 365501.
- Fan, Z.; Ouyang, J.; Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Advanced Electronic Materials 2019, 5, 1800769.
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669.
- Masoumi, S.; O’Shaughnessy, S.; Pakdel, A. Organic-based flexible thermoelectric generators: From materials to devices. Nano Energy 2022, 92, 106774.
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A Review on Organic Polymer-Based Thermoelectric Materials. Journal of Polymers and the Environment 2017, 25, 1208–1218.
- Guan, X.; Cheng, H.; Ouyang, J. Significant enhancement in the Seebeck coefficient and power factor of thermoelectric polymers by the Soret effect of polyelectrolytes. Journal of Materials Chemistry A 2018, 6, 19347–19352.
- Yang, S.; Cho, K.; Park, Y.; Kim, S. Bendable thermoelectric generators composed of p- and n-type silver chalcogenide nanoparticle thin films. Nano Energy 2018, 49, 333–337.
- Kim, S. J.; Lee, H. E.; Choi, H.; Kim, Y.; We, J. H.; Shin, J. S.; Lee, K. J.; Cho, B. J. High-Performance Flexible Thermoelectric Power Generator Using Laser Multiscanning Lift-Off Process. ACS Nano 2016, 10, 10851–10857.
- Zhao, D.; Wang, H.; Khan, Z. U.; Chen, J. C.; Gabrielsson, R.; Jonsson, M. P.; Berggren, M.; Crispin, X. Ionic thermoelectric supercapacitors. Energy & Environmental Science 2016, 9, 1450–1457.
- Yang, K.; Cho, K.; Yang, S.; Park, Y.; Kim, S. A laterally designed all-in-one energy device using a thermoelectric generator-coupled micro supercapacitor. Nano Energy 2019, 60, 667–672.
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. Thermally chargeable supercapacitor using a conjugated conducting polymer: Insight into the mechanism of charge-discharge cycle. Chemical Engineering Journal 2019, 373, 493–500.
- Park, Y.; Cho, K.; Kim, S. Vertical all-in-one energy systems constructed with thermoelectric generators and microsupercapacitors. Journal of Power Sources 2021, 510, 230402.
- Liu, Z.; Cao, X.; Wang, B.; Xia, M.; Lin, S.; Guo, Z.; Zhang, X.; Gao, S. Coupling thermoelectricity and electrocatalysis for hydrogen production via PbTePbS/TiO2 heterojunction. Journal of Power Sources 2017, 342, 452–459.
- Liu, K.; Li, K.; Yang, Z.; Zhang, C.; Deng, J. An advanced Lithium-ion battery optimal charging strategy based on a coupled thermoelectric model. Electrochimica Acta 2017, 225, 330–344.
- Richards, G.; Gemmen, R. S.; Williams, M. C. Solid – state electrochemical heat engines. International Journal of Hydrogen Energy 2015, 40, 3719–3725.
- Liao, G.; Jiang, K.; Zhang, F.; E, J.; Liu, L.; Chen, J.; Leng, E. Thermal performance of battery thermal management system coupled with phase change material and thermoelectric elements. Journal of Energy Storage 2021, 43, 103217.
- Nguyen, H. Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Conversion and Management 2020, 204, 112328.
- Geffroy, C.; Lilley, D.; Parez, P. S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096.
