Peripheral artery disease (PAD) increases the risk of diabetes, while diabetes increases the risk of PAD, and certain symptoms in each disease increase the risk of contracting the other. The phenotypic manifestations of atherosclerosis vary in each individual and throughout the body; it is not fully understood why plaque formation has such a heterogeneous distribution, although different arterial systems are correlated. Moreover, risk factors (such as diabetes mellitus, hypertension, smoking, etc.) undoubtedly aggravate atherogenesis and cardiovascular mortality through a dual risk: firstly, the intrinsic risk of the underlying disease; secondly, it increases the risk of atherosclerosis in various target organs.
- guidelines
- PAD
- antiplatelet therapy
1. Introduction
2. Management: Differences between Guidelines
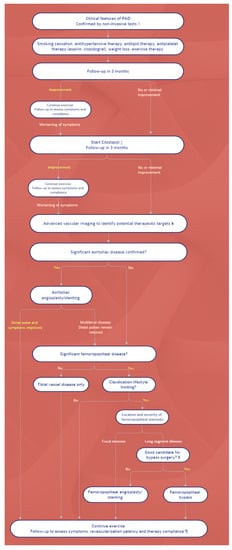
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19169801
References
- Wong, N.D.; Gransar Shaw, L.; Polk, D.; Moon, J.H.; Miranda-Peats, R.; Hayes, S.W.; Thomson, L.E.; Rozanski, A.; Friedman, J.D.; Berman, D.S. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc. Imaging 2009, 2, 319–326.
- Achim, A.; Kákonyi, K.; Nagy, F.; Jambrik, Z.; Varga, A.; Nemes, A.; Chan, J.S.K.; Toth, G.G.; Ruzsa, Z. Radial Artery Calcification in Predicting Coronary Calcification and Atherosclerosis Burden. Cardiol. Res. Pract. 2022, 2022, 5108389.
- Homorodean, C.; Leucuta, D.C.; Ober, M.; Homorodean, R.; Spinu, M.; Olinic, M.; Tataru, D.; Olinic, D.M. Intravascular ultrasound insights into the unstable features of the coronary atherosclerotic plaques: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13671.
- Achim, A.; Marc, M.; Ruzsa, Z. Surgical Turned-Downed CHIP Cases-Can PCI Save the Day? Front. Cardiovasc. Med. 2022, 9, 872398.
- The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am. J. Cardiol. 1995, 75, 894–903.
- Jones, W.S.; Baumgartner, I.; Hiatt, W.R.; Heizer, G.; Conte, M.S.; White, C.J.; Berger, J.S.; Held, P.; Katona, B.G.; International Steering Committee and Investigators of the EUCLID Trial; et al. Ticagrelor Compared with Clopidogrel in Patients with Prior Lower Extremity Revascularization for Peripheral Artery Disease. Circulation 2017, 135, 241–250.
- Low Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; EUCLID Trial Executive Committee and Investigators; et al. Cardiovascular and Limb Outcomes in Patients with Diabetes and Peripheral Artery Disease: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284.
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725.
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; ESC Scientific Document Group; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816.
- Balletshofer, B.; Ito, W.; Lawall, H.; Malyar, N.; Oberländer, Y.; Reimer, P.; Rittig, K.; Zähringer, M. Position Paper on the Diagnosis and Treatment of Peripheral Arterial Disease (PAD) in People with Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127 (Suppl. 1), S105–S113.
- Törngren, K.; Eriksson, S.; Arvidsson, J.; Falkenberg, M.; Johnsson, Å.A.; Sjöberg, C.; Lagerstrand, K.; Nordanstig, J. A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls. J. Clin. Med. 2021, 10, 3643.
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339.
- Olinic, D.M.; Stanek, A.; Tătaru, D.A.; Homorodean, C.; Olinic, M. Acute Limb Ischemia: An Update on Diagnosis and Management. J. Clin. Med. 2019, 8, 1215.
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; COMPASS Investigators; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004.
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510.
