Gastric cancer remains the fifth most common cancer worldwide, with over a million new cases having being diagnosed in 2020 alone, and it is the third leading cause of cancer-related deaths worldwide, accounting for approximately 768,000 deaths in 2020 [
1]. Gastric cancer is associated with a number of factors, including the host’s genetics,
Helicobacter pylori infection, diet, socioeconomic status, and lifestyle [
2]. Gastric cancer has a poor prognosis, with the majority of patients being diagnosed at an advanced stage of the disease, particularly in developing countries [
3]. The treatment of gastric cancer includes surgery for early gastric cancer and chemotherapy for advanced gastric cancer [
4]. The immune response of the host is critical for the immune surveillance of cancer and immunoediting [
5]. The importance of the immune system in cancer surveillance has been shown in animal models, where mice deficient in the interleukin-12β2 receptor, which is an important receptor in the interferon-γ pathway, develop lung cancer [
6]. In a human study, some patients with lymphoma had a mutation in the perforin gene [
7]. Cancer immunoediting is the interaction between immune cells and tumor cells for the protection, formation, or enhancement of the cancer cells. Cancer immunoediting can be divided into three phases: (1) the elimination phase, (2) the equilibrium phase, and (3) the escape phase [
8]. In the elimination process, the immune system uses innate and adapted immunity to recognize and kill cancer cells, with the elimination mechanism involving cytokines such as interferon γ [
9] and immune cells such as natural killer cells, T cells, and B cells [
10]. Studies have shown that the equilibrium stage between cancer cells and immune cells can either suppress or enhance tumor development [
11]. Furthermore, the escape process includes the loss of major histocompatibility complex (MHC) class I from cancer cells, which decreases the capacity of cytotoxic cells to detect cancer cells, as well as a rise in oncogene mutations and the development of a tumor microenvironment [
12]. Immunotherapy is the manipulation of the immune system as a cancer treatment, and it presents as one of the most attractive new strategies for the treatment of gastric cancer. Examples of immunotherapy include cancer vaccines for the activation and development of T cells, monoclonal antibodies, and RNA-based vaccines [
13]. In gastric cancer, the monoclonal antibody trastuzumab, which targets HER2, has been employed together with chemotherapy agents, with promising outcomes of good prognosis in the patients [
14]. Moreover, the use of cytotoxic cells that express CD3
+CD56
+ and that are stimulated with cytokines show promising outcomes in patients with gastric cancer [
15]. Thus, an understanding of how immune cells are stimulated for the treatment of gastric cancer is pertinent to designing strategies for the manipulation of cells as future medical strategies for gastric oncology. However, the roles of immune cells in gastric cancer pathogenesis remain to be elucidated.
2. Functions of Immune Cells in Gastric Cancer
2.1. T Cells
T cells have different categories: cytotoxic T cells (CD8+ cells), T helper cells (CD4+ cells), and regulatory T cells. However, there are T cell categories that are yet to be characterized or discovered for their functions. Several types of T helper cells, based on their cytokine secretion, include T helper cell 1 (Th1), T helper cell 2 (Th2), T helper cell 9 (Th9), T helper cell 17 (Th17), T helper cell 22 (Th22), and T helper cells with high levels of expression for forkhead box 3 (FOXP3) and CD25 [
52]. For instance, Th1 produces interferon-gamma (IFN-γ) and interleukin-2 (IL-2), while Th2 produces interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-6 (IL-6), and interleukin-10 (IL-10). Those cytokines play an essential role in activating cell-mediated immunity and orchestrating B-cell mediated humoral immunity [
53]. Th9 cells secrete interleukin-9 (IL-9), which involves the orchestration of cell-mediated immunity [
54], while Th17 cells produce interleukin-17 (IL-17), interleukin-21 (IL-21), and interleukin-22 (IL-22), which are pertinent in combating extracellular pathogens [
55]. On the other hand, Th22 secretes interleukin-22 (IL-22), which is an essential activator of innate immunity in epithelial cells [
56]. Generally, the functions of T cytotoxic cells include the neutralization of host cells that are infected with intracellular pathogens, such as viruses and some bacteria, and killing cancer cells. In contrast, the function of T helper cells primarily involves the crucial neutralization of cells that have been infected with intracellular pathogens through the mediation of B cells to produce antibodies, the activation of macrophages for bactericidal activity, and the facilitation of other immune cells’ activities in orchestrating immune defense.
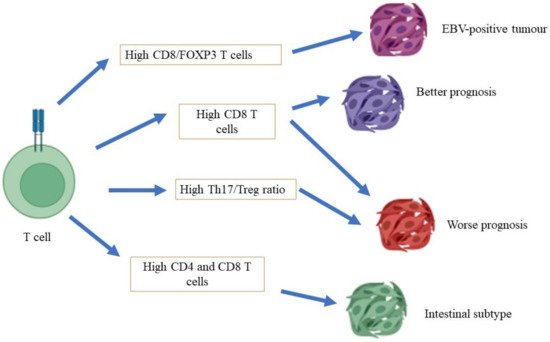
Conflicting reports have been made with regard to the role of T cytotoxic cells in the carcinogenesis of gastric cancer (
Figure 1). At one end of the spectrum, the distinct expression of T cytotoxic cells has been associated with a different prognosis of gastric cancer, in which a high density of T cells in patients with gastric cancer is associated with a poor prognosis of gastric cancer. A study conducted by Thompson et al. [
57] showed that patients with a poor prognosis demonstrated a higher infiltration of CD8+ T cells in gastric tumors compared to patients with a better prognosis. They also found that gastric cancer cells that had been isolated from patients with a poor prognosis also expressed high levels of programmed cell death ligand 1 (PDL-1), which is a critical ligand that deactivates T cells via the stimulation of T regulatory cells, and subsequently encourages autoimmunity and the incidence of autoimmune diseases [
58]. Of note, a high expression of CD8+ T cells has been shown in mixtures of cells that have been isolated from the tumors of patients with gastric cancer who were infected with the Epstein–Barr virus (EBV) [
59]. At the other end of the spectrum, a study conducted by Lee et al. [
60] showed that a high level of infiltration by CD8+ T cells in patients with gastric cancer was associated with a better rate of survival than for patients with a low degree of infiltration by CD8+ T cells. In the mouse model, a high infiltration of CD8+ T cells, with the absence of CD4+ T cells, has been associated with severe gastritis. This finding concludes that CD4+ T cells are vital for the surveillance of CD8+ T cell activity [
61].
Figure 1. Role of T cells in gastric cancer.
The balance of Th1/Th2 is paramount for
H. pylori infection, and polymorphonuclear cells of the gastric mucosa from mice infected with
H. pylori have been found to secrete a high level of IFN-γ and IL-4. Additionally, mice deficient in IL-4 demonstrate a phenotype of severe gastritis compared to wild-type mice, indicating the importance of cytokine secretion by Th1 and Th2 in orchestrating gastric carcinogenesis [
62]. Mice lacking Th1, IL-2, and IFN-γ, but not Th2 and IL-4, were susceptible to
H. pylori infection. However, cytokines released by Th1 cells, namely IL-12 and IFN-γ, are essential in the development of chronic gastritis [
63]. In patients with gastric cancer, the gene expression of IFN-γ (the Th1 cytokine) was lower than the expression of IL-4 (the Th2 cytokine), suggesting that Th2 cells were more prominent than Th1 [
64].
T regulatory (Treg) cells are CD4
+ T cells with characteristics such as a high level of CD4, FOXP3, and CD25 expression. The primary functions of Treg cells include the prevention of autoimmune diseases and the deactivation of allergy reactions [
65]. The profiling of T cells from the peripheral blood of patients with gastric cancer with different rates of prognosis revealed a high density of Treg cells in patients with a worse prognosis than those of patients with a good prognosis and of healthy subjects, in which Treg cells (CD4
+/CD25
+) expressed a higher level of IL-10 secretion than CD4
+/CD25
− cells [
66]. A different result was observed in another study, in which a high density of Treg in patients with gastric cancer was associated with a better prognosis (a low TNM stage and a low rate of tumor invasion) than in patients with gastric cancer with a low density of Treg [
67]. A Treg cell subset with a high level of T-cell receptor co-stimulatory receptor (ICOS) expression was isolated from patients with late-stage gastric cancer and not from patients with early-stage gastric cancer, apart from the high rate of
H. pylori infection in patients with the presence of this Treg subset [
68]. On the other hand, an imbalance between Th17 and Treg has been associated with gastric cancer development. A high Th17/Treg ratio was found in patients with gastric cancer with high lymph node metastasis, and high levels of Th17 and Treg cells, compared to patients in the control group [
69].
2.2. B Cells
B cells function as antigen-presenting cells, and they can differentiate into plasma cells to produce antibodies and to secrete cytokines such as IL-6, IFN-γ, and tumor necrosis α (TNF-α), for the development of CD4+ effector and memory T cells [
70]. A subset of B regulatory cells (Breg) has been shown to inhibit antitumoral activity that is mediated by T cells and other immune cells, namely effector T cells, natural killer cells, and tumor-associated macrophages [
71]. A comprehensive immunophenotype of gastric cancer cells using an antibody microarray showed a higher expression of B-cell markers, including CD19, CD38, and surface immunoglobulin (sIg), in patients with
H. pylori-infected gastric cancer than in patients without infections [
72].
2.3. Macrophages
Macrophages are phagocytes that play a pivotal role in the clearance of erythrocytes and tissue modeling [
73]. Furthermore, these cells also have an essential role in recognizing pathogens via receptor recognition and the neutralization of apoptotic cells [
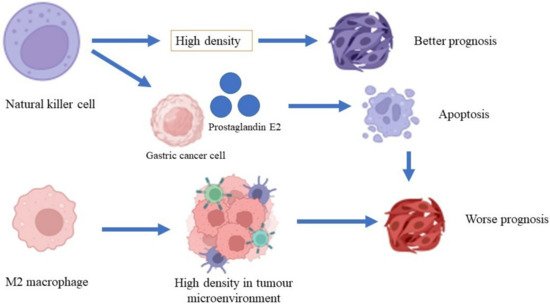
74]. A high density of macrophages is associated with a low rate of survival in patients with gastric cancer and increased gastric tumor invasiveness through the β-catenin pathway (
Figure 2) [
75]. A high density of tumor-associated macrophages (TAM) of phenotype M2 was found in patients with gastric cancer with invasive gastric peritoneum compared to patients without invasive gastric peritoneum. Additionally, a gastric cancer cell line co-cultured with the TAM phenotype M2 also showed increased degrees of invasiveness and cell division in the xenographic model [
76]. Osteopontin, a protein that is involved in inflammation and cancer pathogenesis [
77], recruits macrophages into the tumor microenvironment and polarizes macrophages to the M2 phenotype, which facilitates gastric cancer development [
78]. On the other hand, macrophages induce invasiveness, migration, and gastric cancer cell movement through epidermal growth factor and the phosphorylation of Akt, Erk ½, and c-Src [
79].
H. pylori-infected macrophages demonstrated the activation of immune-regulated genes such as Cd44, Cd40, Cd86, and Cd274, and the inhibition of macrophage cell line division, primary macrophage cells, and the transcription of genes that are involved in DNA synthesis and the cell cycle [
80].
Figure 2. Roles of macrophages and natural killer cells in gastric cancer.
2.4. Natural Killer Cells
Natural killer cells are lymphocytes that are important in mediating innate immunity and adaptive immunity for the surveillance of the immune system against invading intracellular viruses and cancer cells [
81]. Natural killer cells can recognize cancer cells because they express natural killer group-2 (NKG2D), which is expressed by cancer cells [
82]. The expressions of CD antigens such as CD56 and CD16 play essential roles in mediating the activation of immune cells, including macrophages, T cells, and dendritic cells [
83]. A study conducted by Ishigami et al. [
84] revealed that a high density of natural killer cells in patients with gastric cancer is associated with a better prognosis of gastric cancer compared to a low density of natural killer cells. A lysis activity that is greater than 25% in natural killer cells is significantly associated with a better five-year survival rate compared to lysis activity that is less than 25% [
85]. A high apoptosis rate for natural killer cells was shown in patients with advanced-stage gastric cancer. However, the rate of apoptosis was reduced after the patients underwent gastrectomy [
86]. Gastric cancer cells produce prostaglandin E2, which inhibits natural killer cell division and encourages apoptosis [
87].
2.5. Dendritic Cells
Dendritic cells, or antigen-presenting cells, are master regulators of innate immunity and adaptive immunity because they can present antigens to major histocompatibility complex (MHC) class I and MHC class II. Immature dendritic cells express low levels of CD54, CD58, CD80, CD86, CD40, CD25, CD83, and IL-12, whereas mature dendritic cells express those CD antigens at high levels. IL-12 is an essential cytokine for activating natural killer cells, T cells, and B cells [
88]. There are several types of dendritic cells, including myeloid dendritic cells, plasmacytoid dendritic cells, Langerhans cells, microglia cells, and CD14+ dendritic cells, which are based on the cell markers that are expressed on their surfaces [
89]. In ovarian cancer cells, dendritic cells express high levels of B7-H1 and PD-1, which inhibit T-cell activation and deactivate the function of dendritic cells in recognizing tumors [
90]. Factors that are secreted by tumor cells inhibit the differentiation of dendritic cells, which deactivates their antitumoral properties [
91]. Furthermore, factors that are produced by tumor cells leads to the high expression of CD11b, which reduces the expression of MHC class II and is associated with a worse prognosis in patients with gastric cancer [
92].
3. Immune Checkpoints in Gastric Cancer
Immune checkpoints are essential to maintain the self-tolerance of the immune system to body cells and prevent autoimmune reactions. Tumour cells exploit the immune checkpoint molecules to their advantage by downregulating the surveillance of immune cells toward the cancer cells [
93]. Immune checkpoint molecules are often expressed on immune cells as master regulators to keep the immune system in check. Immune checkpoint molecules that have been extensively studied include programmed cell death receptor-1 (PD-1), programmed cell death receptor ligand-1 (PD-L1), programmed cell death receptor ligand-2 (PD-L2), lymphocyte activation-gene-3 (LAG-3), cytotoxic T lymphocyte antigen-4 (CTLA-4), B and T lymphocyte attenuator (BTLA) [
94]. Furthermore, an immune checkpoint score based on twenty immune checkpoints has been studied and can be applied to predict the prognosis of gastric cancer patients [
95]. Cancer cells express PD-L1 to escape immune surveillance through binding to PD-1 expressed on activated immune cells, namely T cells, monocytes, macrophages, and natural killer cells. The binding inhibits paramount immune signal transduction pathways and renders immune cells to be unresponsive to cancer cells and causes the downregulation of immune cell proliferation, cytokine secretion, and activation [
96].
Treatments of gastric cancer include curative resection and adjuvant chemotherapy depending on the staging of gastric cancer [
97]. Immunotherapy through the identification of immune checkpoints that can be manipulated to attack cancer cells has emerged as an alternative treatment for gastric cancer. Immune checkpoint inhibitors have been introduced to activate immune cells in gastric cancer patients as part of immunotherapy. Several monoclonal antibodies, namely nivolumab and pembrolizumab, that target the PD-1/PD-L1 immune checkpoint have undergone clinical trial phases with promising results. In a phase 3 clinical trial conducted among gastric cancer patients at an advanced stage from East Asian countries (Japan, South Korea, and Taiwan), the administration of nivolumab increased median follow-up in surviving patients, median overall survival, and the 1-year overall survival rate in the nivolumab group compared to the placebo group. However, adverse events were more frequently observed in the group that received nivolumab than placebo [
98]. Another monoclonal antibody that targets the PD-1/PD-L1 checkpoint is pembrolizumab. A phase 1b clinical trial conducted among advanced gastric cancer patients who were positive for PD-L1 found that pembrolizumab had manageable toxicity safety among patients and elicited antitumoral activity against gastric cancer [
99]. Another drug that targets the PD-1 checkpoint is avelumab. A phase 3 clinical trial that involved advanced gastric cancer patients found that avelumab did not improve overall survival and progression-free survival of the patients compared to chemotherapy, although the toxicity of avelumab was more manageable than chemotherapy [
100]. A combination of monoclonal antibodies that target PD-1 (nivolumab) and CTLA-4 (ipilimumab) have also been used in clinical trials. Recent global phase 3 clinical trials (CheckmMate 649 study) that compared the combination of nivolumab and ipilimumab to that of nivolumab and chemotherapy revealed that the combination of nivolumab and chemotherapy resulted in better overall survival of the patients than that of nivolumab and ipilimumab [
101].