Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The three dehydrogenase enzymes involved in the CO2 to methanol conversion are: Formate dehydrogenase, Formaldehyde dehydrogenase and Alcohol dehydrogenase.
- CO2 reduction
- cofactor regeneration
- enzyme immobilization
1. The Dehydrogenases
The three dehydrogenase enzymes involved in the CO2 to methanol conversion are: Formate dehydrogenase, Formaldehyde dehydrogenase and Alcohol dehydrogenase. Dehydrogenases are enzymes that catalyze the “proton coupled to electron transfer” (PCET, H+ + e−) from a molecule that acts as “electron + proton” donor (reductant) to another one that acts as an acceptor (oxidant). During the redox reaction, NADPH/NADP+ or NADH/NAD+ (nicotinamide adenine dinucleotide) is employed as an essential cofactor. The three enzymes above manage the reduction in the oxidation state of carbon dioxide to carbon-based energy-carrier resources. [1]
2. Formate Dehydrogenase
Formate dehydrogenases are categorized as metal-independent and metal-dependent based on the presence of metals (molybdenum-Mo or tungsten-W) in the active sites. The former catalyzes the reaction from HCO2H to CO2 irreversibly; the latter catalyzes the reduction of CO2 to HCO2H reversibly and, because of this, is also employed in the enzymatic CO2 to HCO2H conversion. [2] The redox potential for the enzymatic reduction of CO2 to HCO2− is E°ʹ = −420 mV, but both types of Formate dehydrogenase (FateDH) mainly catalyze HCO2− oxidation, hence their dehydrogenase designation. The difference in catalytic reaction reversibility is due to differences in the catalytic reaction mechanism and the enzyme structure governing the energy reorganization during catalysis. [3] Among these FateDHs, the one extracted from Candida boidinii (EC 1.2.1.2, CbFateDH) is commercially available and can be easily handled as a catalyst for CO2 reduction. CbFateDH is a homodimer [79 kDa (6 nm × 6 nm × 10 nm)] with two independent active sites catalyzing the NAD+-dependent oxidation of formate to CO2 via an irreversible hydride transfer from formate to NAD+. [4]
The conversion of CO2 to formate is a process thermodynamically unfavorable and which somehow needs to be “helped” to happen. Table 1 shows the Km values with reference to the substrates of the reaction in the two possible directions. The Km is the index of the affinity between enzyme and substrate: the lower its value, the higher the affinity of the substrate for the enzyme. As reported in Table 1, such value is lower for CO2 than for HCO2−, confirming that the formate oxidation reaction is favored. Nevertheless, the formate reduction reaction can be “forced” to take place by working with an excess of substrate and cofactor, i.e., with high amounts of NADH and CO2.
Table 1. Km values for the CO2 reduction–formate oxidation reactions.
While for NADH, the excess is reached by adding the cofactor in the solution, for CO2, it is not so simple due to its low solubility and its interaction with water. Moreover, one must consider that depending on the pH of the solution, CO2 in water is found in three forms: carbon dioxide, hydrogencarbonate, and carbonate, as shown in Figure 1.
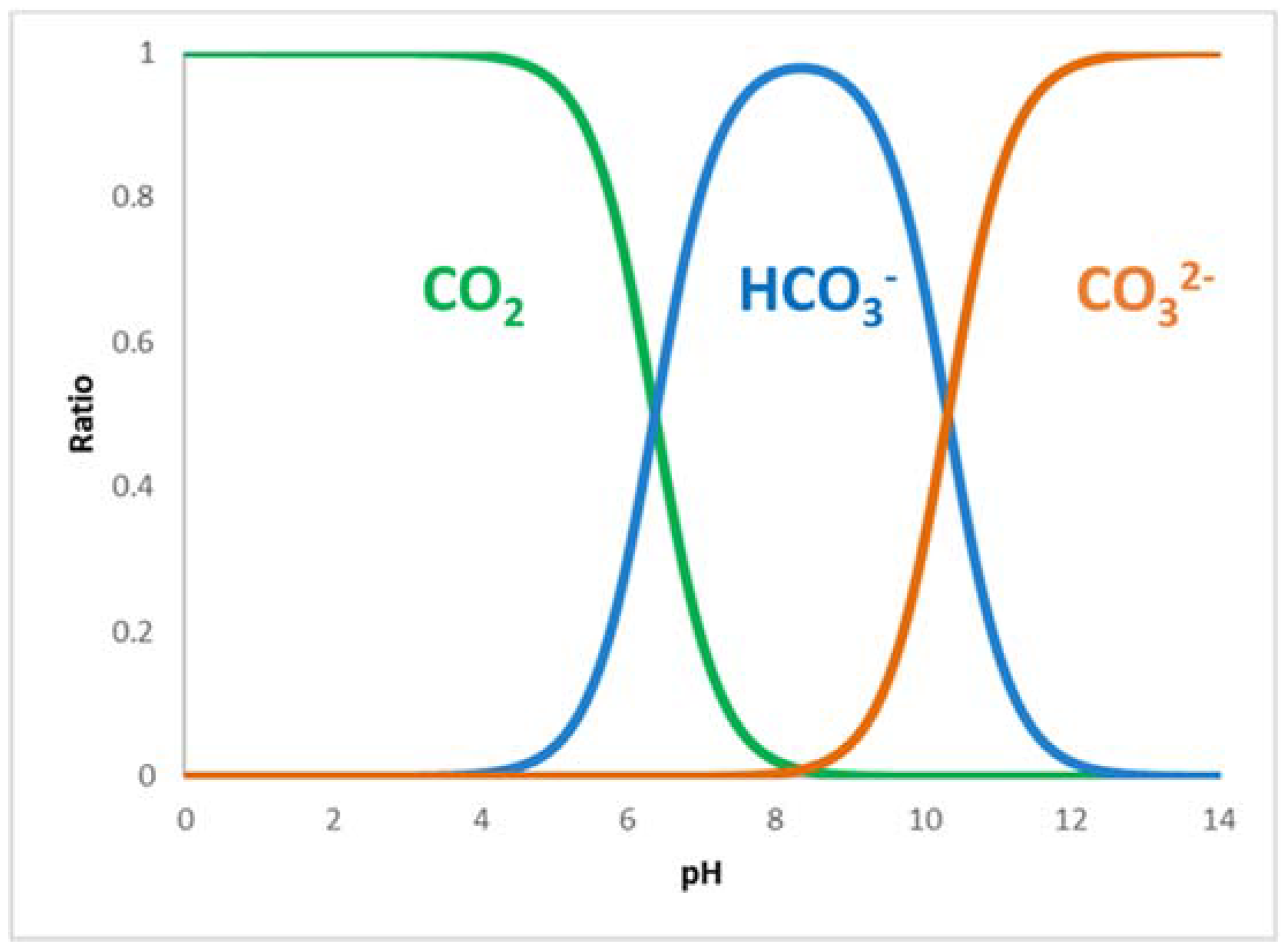
Figure 1. Distribution of species derived from CO2 according to pH in water solution. [6]
There are conflicting opinions in the literature on the role of the CO2 hydration reaction. Sato et al. [7] affirmed that as carbonate and hydrogencarbonate concentrations increase in solution, there is a suppression of the formate formation reaction due to the fact that such species are competitive inhibitors of carbon dioxide for the formate production with CbFateDH. Other authors, see Wang et al.,[8] consider hydrogencarbonate to be the active species, and in some cases (Cazelles et al. [5]), KHCO3 is dissolved in water rather than bubbling CO2 for better reproducibility. In order to increase the presence of CO2 in the solution, this is left to bubble for at least half an hour before starting the reaction, and in some cases, a pressurized system is also used. However, the low solubility of CO2 remains one of the reasons for the low yields of such a reaction. In order to improve the yield, a fourth enzyme is added to the system, i.e., Carbonic anhydrase, which very quickly (kCat = 106 s−1) catalyzes the hydration–dehydration of CO2. (Equation (1))
CO2 + H2O ⇋H+ + HCO3− (1)
According to Wang et al., [8] with the catalysis of CA, CO2 molecules are rapidly hydrated and transformed into hydrogencarbonate, which is converted into formate, while the cofactor NADH is oxidized. Another possibility to increase CO2 availability is to use materials such as MOFs able to both immobilize enzymes and absorb CO2.
Although Formate dehydrogenase from C. boidinii is the most widely used enzyme for testing the reduction reaction, FateDHs are also derived from other microorganisms and have been tested for their ability to reduce CO2. Nielsen et al.[9] compared the catalytic efficiency of Formate dehydrogenase of various organisms by highlighting the type of electron donor, the Km for CO2, and the working conditions. Amongst the various enzymes, the activity of DdFateDH (Desulfovibrio desulfuricans) is particularly interesting, with a Km of 0.02 mM and a catalytic efficiency Kcat/Km of 2968. [10]
Other FateDHs such as, for example, FateDH from Thiobacillus sp. KNK65MA,[11] FateDH from Myceliophthora thermophila,[12] FateDH from Clostridium carboxidivorans,[13] on the other hand, have a low Km (0.95, 0.44, and 0.05 mM, respectively) but present a not very high catalytic efficiency (Kcat/Km = 0.34, 0.23, 1.6, respectively).
3. Formaldehyde Dehydrogenase
The second step of the reaction is the reduction of formate to formaldehyde. The enzyme involved in such a reaction is Formaldehyde dehydrogenase. Again, as observed in the first step, the enzyme has more affinity for formaldehyde than formate, and this affects the reaction yield (Table 2). The kinetic parameters of FaldDH for the reduction reaction (HCO2H → HCHO) have not been determined yet, mainly due to the difficulty of measuring the reaction rates at different formic acid concentrations while keeping the pH constant. [14] Formaldehyde dehydrogenase (FaldDH, EC 1.2.1.46) used in the reduction of formic acid is extracted from Pseudomonas putida and is a homo-tetramer of approximately 168 kDa [4 × 42 kDa (5 nm × 6 nm × 10 nm)]. [5]
Table 2. Km values for the formate reduction–formaldehyde oxidation reactions.
It was shown that the formate reduction step in the enzymatic cascade from CO2 to methanol is the bottleneck of the reaction, as this enzyme has low activity and is sensitive to pH, substrate, and product concentration. [5],[14],[15] Luo et al. [16] reported that if a minimum concentration of 10 mM of formate is not reached, the reaction does not proceed, and higher concentrations at the same time do not improve the speed of the reaction. Therefore, they defined 10 mM as the optimum concentration for the reduction to occur. Such information is obtained by a step-by-step study of the cascade reaction. Given this, it is possible to state that in the cascade reaction, the slow accumulation of formate affects the low yield of the second reduction step from formate to formaldehyde. Starting from formate instead of CO2 would, thus, help the process.
4. Alcohol Dehydrogenase
The third enzyme involved in this reaction is Alcohol dehydrogenase. Alcohol dehydrogenase is present in many organisms, but the mainly used is the one from Saccharomyces cerevisiae (Alcohol dehydrogenase (ADH), EC 1.1.1.1, a homo tetramer of about 141–151 kDa with a size of 7 nm × 10 nm × 11 nm), which is commercially available. ADH normally acts on primary or secondary alcohols, but the enzyme oxidizes methanol much more poorly than ethanol. In the cascade reaction, it is used in the final step of the multienzyme process, i.e., the reversible reduction of formaldehyde to methanol. [17] The forward reaction (formaldehyde → methanol) is much more efficient than the reverse one (methanol → formaldehyde) given the affinity of the substrates for the enzyme (see the km value in Table 3); the reduction in formaldehyde is considered almost irreversible. This step is the only one favored in the direction of the cascade reaction from CO2 to methanol. However, ADH is not a very stable enzyme for industrial use. Indeed, when the three dehydrogenases catalyzing the cascade reaction from CO2 to CH3OH were employed as free enzymes, ADH was by far the least stable one. [18]
Table 3. Km values for the formaldehyde reduction–methanol oxidation reactions.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27154913
References
- Jiafu Shi; Yanjun Jiang; Zhongyi Jiang; Xueyan Wang; Xiaoli Wang; Shaohua Zhang; Pingping Han; Chen Yang; Enzymatic conversion of carbon dioxide. Chemical Society Reviews 2015, 44, 5981-6000, 10.1039/c5cs00182j.
- Fauziah Marpani; Manuel Pinelo; Anne S. Meyer; Enzymatic conversion of CO2 to CH3OH via reverse dehydrogenase cascade biocatalysis: Quantitative comparison of efficiencies of immobilized enzyme systems. Biochemical Engineering Journal 2017, 127, 217-228, 10.1016/j.bej.2017.08.011.
- Liliana Calzadiaz-Ramirez; Anne S Meyer; Formate dehydrogenases for CO2 utilization. Current Opinion in Biotechnology 2021, 73, 95-100, 10.1016/j.copbio.2021.07.011.
- Qi Guo; Lokesh Gakhar; Kyle Wickersham; Kevin Francis; Alexandra Vardi-Kilshtain; Dan T. Major; Christopher M. Cheatum; Amnon Kohen; Structural and Kinetic Studies of Formate Dehydrogenase from Candida boidinii. Biochemistry 2016, 55, 2760-2771, 10.1021/acs.biochem.6b00181.
- Rémi Cazelles; Jullien Drone; François Fajula; Ovidiu Ersen; Simona Moldovan; Anne Galarneau; Reduction of CO2 to methanol by a polyenzymatic system encapsulated in phospholipids–silica nanocapsules. New Journal of Chemistry 2013, 37, 3721-3730, 10.1039/c3nj00688c.
- Garrels, R.; Charles L. C.. Solutions, Minerals, and Equilibria; 1st edition; ISBN 0877353336; Harper & Row, Eds.; Freeman, Cooper & Company: New York, 1965; pp. 1-400.
- Ryohei Sato; Yutaka Amao; Can formate dehydrogenase from Candida boidinii catalytically reduce carbon dioxide, bicarbonate, or carbonate to formate?. New Journal of Chemistry 2020, 44, 11922-11926, 10.1039/d0nj01183e.
- Xiaoli Wang; Zheng Li; Jiafu Shi; Hong Wu; Zhongyi Jiang; Wenyan Zhang; Xiaokai Song; Qinghong Ai; Bioinspired Approach to Multienzyme Cascade System Construction for Efficient Carbon Dioxide Reduction. ACS Catalysis 2014, 4, 962-972, 10.1021/cs401096c.
- Christian Førgaard Nielsen; Lene Lange; Anne S. Meyer; Classification and enzyme kinetics of formate dehydrogenases for biomanufacturing via CO2 utilization. Biotechnology Advances 2019, 37, 107408, 10.1016/j.biotechadv.2019.06.007.
- Luisa B. Maia; Luis Fonseca; Isabel Moura; José J. G. Moura; Reduction of Carbon Dioxide by a Molybdenum-Containing Formate Dehydrogenase: A Kinetic and Mechanistic Study. Journal of the American Chemical Society 2016, 138, 8834-8846, 10.1021/jacs.6b03941.
- Hyunjun Choe; Jeong Chan Joo; Dae Haeng Cho; Min Hoo Kim; Sang Hyun Lee; Kwang-Deog Jung; Yong Hwan Kim; Efficient CO2-Reducing Activity of NAD-Dependent Formate Dehydrogenase from Thiobacillus sp. KNK65MA for Formate Production from CO2 Gas. PLOS ONE 2014, 9, e103111, 10.1371/journal.pone.0103111.
- Nilay Altaş; Aşkın Sevinç Aslan; Ersin Karataş; Evangelia Chronopoulou; Nikolaos E. Labrou; Barış Binay; Heterologous production of extreme alkaline thermostable NAD + -dependent formate dehydrogenase with wide-range pH activity from Myceliophthora thermophila. Process Biochemistry 2017, 61, 110-118, 10.1016/j.procbio.2017.06.017.
- Apostolos Alissandratos; Hye-Kyung Kim; Hayden Matthews; James E. Hennessy; Amy Philbrook; Christopher J. Easton; Clostridium carboxidivorans Strain P7T Recombinant Formate Dehydrogenase Catalyzes Reduction of CO 2 to Formate. Applied and Environmental Microbiology 2013, 79, 741-744, 10.1128/aem.02886-12.
- Pegah S. Nabavi Zadeh; Milene Zezzi Do Valle Gomes; Björn Åkerman; Anders E. C. Palmqvist; Förster Resonance Energy Transfer Study of the Improved Biocatalytic Conversion of CO2 to Formaldehyde by Coimmobilization of Enzymes in Siliceous Mesostructured Cellular Foams. ACS Catalysis 2018, 8, 7251-7260, 10.1021/acscatal.8b01806.
- Milene Zezzi Do Valle Gomes; Anders E.C. Palmqvist; Immobilization of formaldehyde dehydrogenase in tailored siliceous mesostructured cellular foams and evaluation of its activity for conversion of formate to formaldehyde. Colloids and Surfaces B: Biointerfaces 2018, 163, 41-46, 10.1016/j.colsurfb.2017.11.069.
- Jianquan Luo; Anne S. Meyer; R.V. Mateiu; Manuel Pinelo; Cascade catalysis in membranes with enzyme immobilization for multi-enzymatic conversion of CO2 to methanol. New Biotechnology 2015, 32, 319-327, 10.1016/j.nbt.2015.02.006.
- Milene Zezzi Do Valle Gomes; Anders E. C. Palmqvist; Influence of operating conditions and immobilization on activity of alcohol dehydrogenase for the conversion of formaldehyde to methanol. New Journal of Chemistry 2017, 41, 11391-11397, 10.1039/c7nj02028g.
- Birgitte Zeuner; Nicolaj Ma; Kasper Berendt; Anne S Meyer; Pavle Andric; Jan Hoffmann Jørgensen; Manuel Pinelo; Immobilization of alcohol dehydrogenase on ceramic silicon carbide membranes for enzymatic CH3 OH production. Journal of Chemical Technology & Biotechnology 2018, 93, 2952-2961, 10.1002/jctb.5653.
This entry is offline, you can click here to edit this entry!
