Wilson’s disease (WD) is an autosomal recessive genetic disorder due to a mutation of the ATP7B gene, resulting in impaired hepatic copper excretion and accumulation in various tissues. Ocular findings are one of the hallmarks of the disease. Many ophthalmological manifestations have been described and new techniques are currently available to improve their diagnosis and to follow their evolution. The most common ocular findings seen in WD patients are Kayser–Fleischer ring (KFR) and sunflower cataracts. Other ocular manifestations may involve retinal tissue, visual systems and eye mobility. Diagnosis and follow-up under decoppering treatment of these ocular findings are generally easily performed with slit-lamp examination (SLE). However, new techniques are available for the precocious detection of ocular findings due to WD and may be of great value for non-experimented ophthalmologists and non-ophthalmologists practitioners.
- Wilson’s disease
- Kayser–Fleischer ring
- eye involvement
- sunflower cataract
- copper
1. Introduction
2. Eye Involvement in Wilson’s Disease
2.1. Cornea Involvement
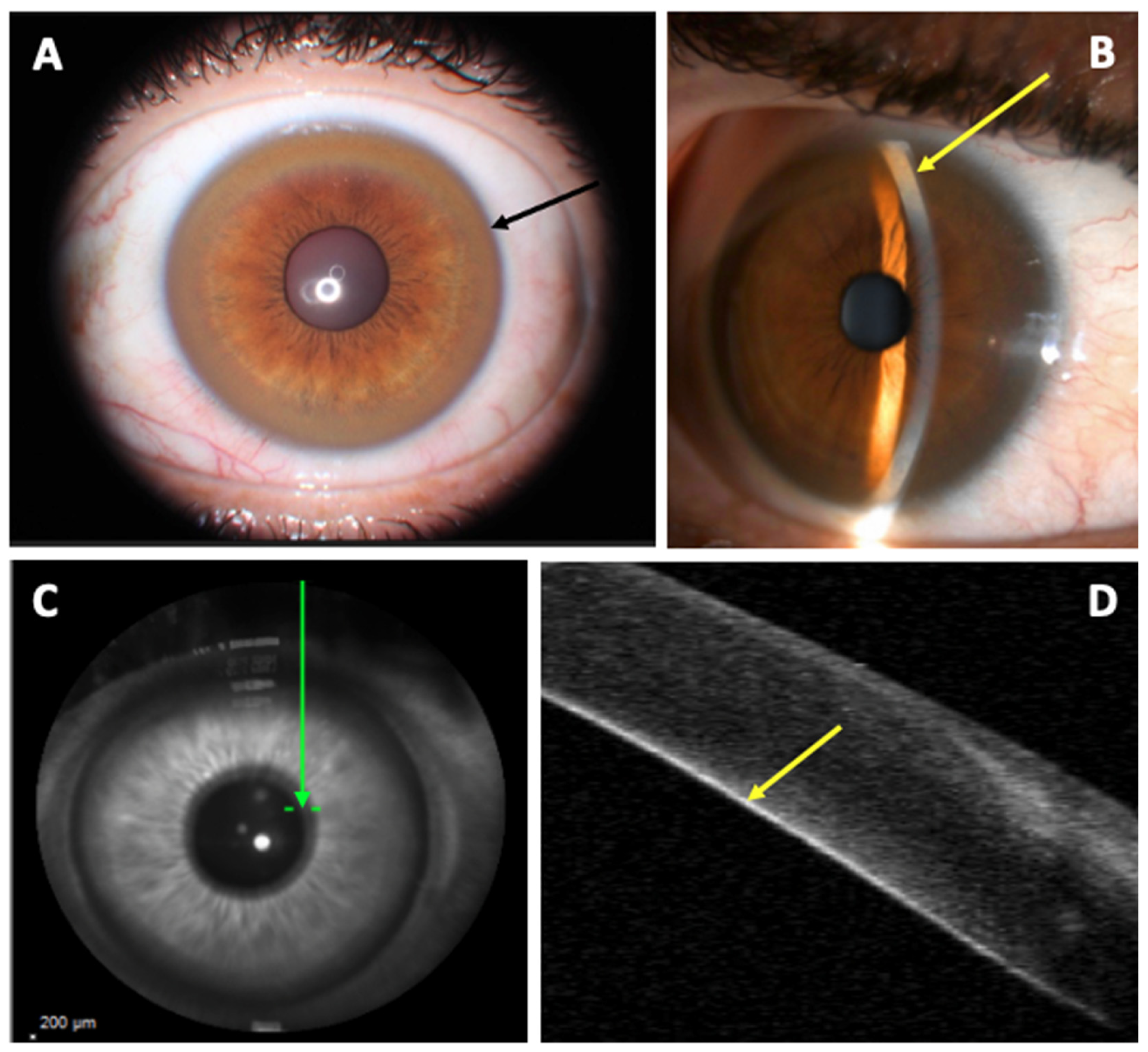
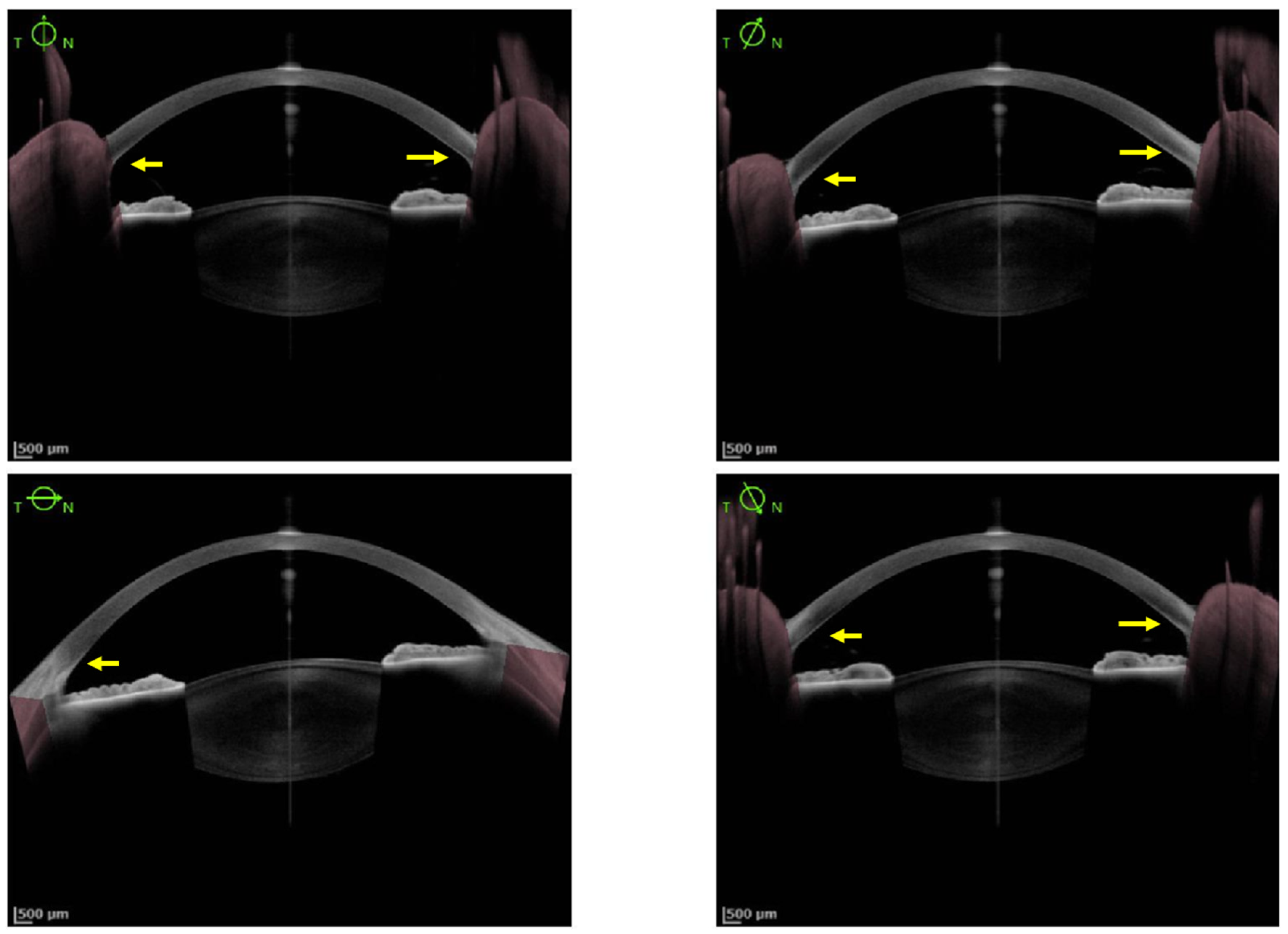
2.2. Lens Involvement
2.3. Macula, Retinal Nerve Fiber Layer and Visual Pathways Involvement
2.4. Eye Mobility
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jcm11092528
References
- Poujois, A.; Woimant, F.; Samson, S.; Chaine, P.; Girardot-Tinant, N.; Tuppin, P. Characteristics and prevalence of Wilson’s disease: A 2013 observational population-based study in France. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 57–63.
- Roberts, E.A.; Schilsky, M.L.; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111.
- Broniek-Kowalik, K.; Dzieżyc, K.; Litwin, T.; Członkowska, A.; Szaflik, J.P. Anterior segment optical coherence tomography (AS-OCT) as a new method of detecting copper deposits forming the Kayser–Fleischer ring in patients with Wilson disease. Acta Ophthalmol. 2019, 97, e757–e760.
- Kelly, C.; Pericleous, M. Wilson disease: More than meets the eye. Postgrad. Med. J. 2018, 94, 335–347.
- Pfeiffer, R.F. Wilson’s Disease. Skull Base 2007, 27, 123–132.
- Fenu, M.; Liggi, M.; Demelia, E.; Sorbello, O.; Civolani, A. Kayser–Fleischer ring in Wilson’s disease: A cohort study. Eur. J. Intern. Med. 2012, 23, e150–e156.
- Prasad, D.; Bhriguvanshi, A. Ocular manifestations of liver disease in children: Clinical aspects and implications. Ann. Hepatol. 2020, 19, 608–613.
- Vitiello, L.; De Bernardo, M.; Nuzio, S.G.; Mandato, C.; Rosa, N.; Vajro, P. Pediatric liver diseases and ocular changes: What hepatologists and ophthalmologists should know and share with each other. Dig. Liver Dis. 2019, 52, 1–8.
- Walshe, J.M. The eye in Wilson disease. QJM 2010, 104, 451–453.
- Członkowska, A.; Litwin, T.; Chabik, G. Wilson Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 142, pp. 101–119. ISBN 978-0-444-63625-6.
- Innes, J.R.; Strachan, I.M.; Triger, D.R. Unilateral Kayser-Fleischer ring. Br. J. Ophthalmol. 1986, 70, 469–470.
- Al-Khaier, A.; Nischal, K.K. The eye in metabolic disease. Hosp. Med. 2003, 64, 609–612.
- Alakus, M.F.; Caglayan, M.; Ekin, N.; Oncul, H.; Arac, E.; Dag, U.; Diri, H. Investigation of corneal topographic and densitometric properties of Wilson’s disease patients with or without a Kayser-Fleischer ring. Eye Vis. 2021, 8, 1–8.
- Gitlin, J.D. Wilson disease. Gastroenterology 2003, 125, 1868–1877.
- Walshe, J.M. The Kayser–Fleischer Ring. Br. J. Hosp. Med. 2014, 75, 2.
- Sridhar, U.; Tripathy, K. Commentary: Kayser-Fleischer-like rings in patients with hepatic disease. Indian J. Ophthalmol. 2021, 69, 1088.
- Langwińska-Wośko, E.; Litwin, T.; Dzieżyc, K.; Członkowska, A. The sunflower cataract in Wilson’s disease: Pathognomonic sign or rare finding? Acta Neurol. Belg. 2015, 116, 325–328.
- Youn, J.; Kim, J.S.; Kim, H.-T.; Lee, J.-Y.; Lee, P.H.; Ki, C.-S.; Cho, J.W. Characteristics of neurological Wilson’s disease without Kayser–Fleischer ring. J. Neurol. Sci. 2012, 323, 183–186.
- Demirkiran, M.; Jankovic, J.; Lewis, R.A.; Cox, D.W. Neurologic presentation of Wilson disease without Kayser-Fleischer rings. Neurology 1996, 46, 1040–1043.
- Medici, V.; Rossaro, L.; Sturniolo, G. Wilson disease—A practical approach to diagnosis, treatment and follow-up. Dig. Liver Dis. 2007, 39, 601–609.
- Willeit, J.; Kiechl, S. Wilson’s disease with neurological impairment but no Kayser-Fleischer rings. Lancet 1991, 337, 1426.
- Imam, L.; Haboubi, H.N. G-Eye: Ocular manifestations of gastrointestinal disease. Front. Gastroenterol. 2019, 11, 162–167.
- Lorincz, M.T. Neurologic Wilson’s disease. Ann. N. Y. Acad. Sci. 2010, 1184, 173–187.
- Couchonnal, E.; Lion-François, L.; Guillaud, O.; Habes, D.; Debray, D.; Lamireau, T.; Broué, P.; Fabre, A.; Vanlemmens, C.; Sobesky, R.; et al. Pediatric Wilson’s Disease: Phenotypic, Genetic Characterization and Outcome of 182 Children in France. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e80–e86.
- Grupchev, D.I.; Radeva, M.N.; Georgieva, M.; Grupcheva, C.N. In vivo confocal microstructural analysis of corneas presenting Kayser-Fleischer rings in patients with Wilson’s disease. Arq. Bras. Oftalmol. 2018, 81, 137–143.
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685.
- Socha, P.; Janczyk, W.; Dhawan, A.; Baumann, U.; D’Antiga, L.; Tanner, S.; Iorio, R.; Vajro, P.; Houwen, R.; Fischler, B.; et al. Wilson’s Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 334–344.
- Sridhar, M.S. Advantages of Anterior Segment Optical Coherence Tomography Evaluation of the Kayser–Fleischer Ring in Wilson Disease. Cornea 2017, 36, 343–346.
- Sridhar, M.S.; Rangaraju, A.; Anbarasu, K.; Reddy, S.P.; Daga, S.; Jayalakshmi, S.; Shaik, B. Evaluation of Kayser–Fleischer ring in Wilson disease by anterior segment optical coherence tomography. Indian J. Ophthalmol. 2017, 65, 354–357.
- Sridhar, M.S.; Pineda, R. Anterior segment optical coherence tomography to look for Kayser-Fleischer rings. Pract. Neurol. 2017, 17, 222–223.
- Telinius, N.; Ott, P.; Hjortdal, J. Detection of Kayser-Fleischer ring using Scheimpflug imaging. Acta Ophthalmol. 2016, 95, e248–e249.
- Zhao, T.; Fang, Z.; Tian, J.; Liu, J.; Xiao, Y.; Li, H.; Chen, B. Imaging Kayser-Fleischer Ring in Wilson Disease Using In Vivo Confocal Microscopy. Cornea 2018, 38, 332–337.
- Ceresara, G.; Fogagnolo, P.; Zuin, M.; Zatelli, S.; Bovet, J.; Rossetti, L. Study of Corneal Copper Deposits in Wilson’s Disease by in vivo Confocal Microscopy. Ophthalmologica 2014, 231, 147–152.
- Rathi, A.; Takkar, B.; Gaur, N.; Maharana, P.K. Optical coherence tomography of the Kayser-Fleischer ring: An ancillary diagnostic tool for Wilson’s disease in children. BMJ Case Rep. 2017, 2017, bcr-2017-220007.
- Siemerling, E.; Oloff, H. Pseudosklerose (Westphal-Strümpell). Klin. Wochenschr. 1922, 1, 1087–1089.
- Litwin, T.; Langwińska-Wośko, E.; Dzieżyc, K.; Członkowska, A. Sunflower cataract: Do not forget Wilson’s disease. Pract. Neurol. 2015, 15, 385–386.
- Langwińska-Wośko, E.; Litwin, T.; Szulborski, K.; Członkowska, A. Optical coherence tomography and electrophysiology of retinal and visual pathways in Wilson’s disease. Metab. Brain Dis. 2015, 31, 405–415.
- Albrecht, P.; Müller, A.-K.; Ringelstein, M.; Finis, D.; Geerling, G.; Cohn, E.; Aktas, O.; Hartung, H.-P.; Hefter, H.; Methner, A. Retinal Neurodegeneration in Wilson’s Disease Revealed by Spectral Domain Optical Coherence Tomography. PLoS ONE 2012, 7, e49825.
- Satishohandra, P.; Naik, K.R. Visual pathway abnormalities Wilson’s disease: An electrophysiological study using electroretinography and visual evoked potentials. J. Neurol. Sci. 2000, 176, 13–20.
- Jung, H.-K.; Choi, S.Y.; Kim, J.-M.; Kim, J.-S. Selective slowing of downward saccades in Wilson’s disease. Park. Relat. Disord. 2013, 19, 134–135.
- Kirkham, T.H.; Kamin, D.F. Slow saccadic eye movements in Wilson’s disease. J. Neurol. Neurosurg. Psychiatry 1974, 37, 191–194.
- Ingster-Moati, I.; Quoc, E.B.; Pless, M.; Djomby, R.; Orssaud, C.; Guichard, J.P.; Woimant, F. Ocular motility and Wilson’s disease: A study on 34 patients. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1199–1201.
- Leśniak, M.; Członkowska, A.; Seniów, J.S. Abnormal antisaccades and smooth pursuit eye movements in patients with Wilson’s disease. Mov. Disord. 2008, 23, 2067–2073.
