Biosensors are powerful analytical tools used to identify and detect target molecules. Electrochemical biosensors, which combine biosensing with electrochemical analysis techniques, are efficient analytical instruments that translate concentration signals into electrical signals, enabling the quantitative and qualitative analysis of target molecules. Electrochemical biosensors have been widely used in various fields of detection and analysis due to their high sensitivity, superior selectivity, quick reaction time, and inexpensive cost. However, the signal changes caused by interactions between a biological probe and a target molecule are very weak and difficult to capture directly by using detection instruments. Therefore, various signal amplification strategies have been proposed and developed to increase the accuracy and sensitivity of detection systems. This review serves as a reference for biosensor and detector research, as it introduces the research progress of electrochemical signal amplification strategies in olfactory and taste evaluation. It also discusses the latest signal amplification strategies currently being employed in electrochemical biosensors for nanomaterial development, enzyme labeling, and nucleic acid amplification techniques, and highlights the most recent work in using cell tissues as biosensitive elements.
- electrochemical biosensors
- olfactory and taste evaluation
- signal amplification strategies
- nanomaterials
- enzymes
- nucleic acid amplification techniques
1. Electrochemical Biosensors
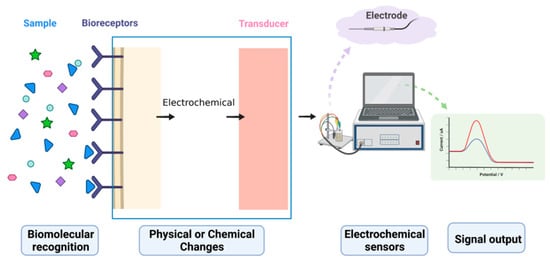
2. Advances in Electrochemical Signal Amplification Strategies for Olfactory and Taste Measurements
2.1. Classical Analytical Techniques for Olfactory and Taste Detection
2.2. Olfactory and Taste Detection Based on Biosensor Technology
2.3. Taste Electrochemical Sensors Based on a Cellular Signal Cascade Amplification System
2.4. Olfactory Electrochemical Sensors Based on a Cellular Signal Cascade Amplification System
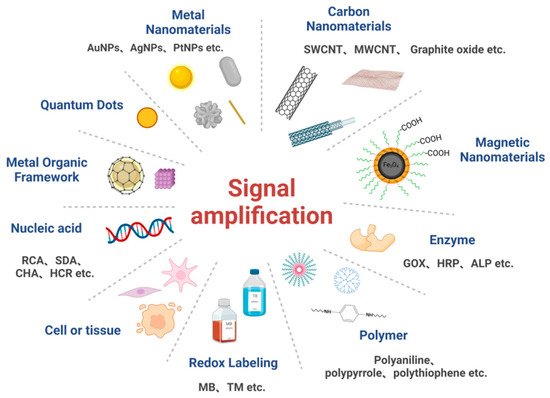
3. Commonly Used Signal Amplification Strategies for Electrochemical Biosensors
3.1. Signal Amplification Strategies Based on Nanomaterials
| Strategies | Examples | Limit of Detections | Linearity Ranges | Ref. |
|---|---|---|---|---|
| Metallic nanomaterials |
Electrochemical aptasensors for ATP detection based on sulfhydryl chemistry and DNA self-assembly techniques and gold nanoparticles | 29.6 aM | ATP:10 fmol/L–1 mmol/L | [63] |
| Electrochemical biosensor based on gold nanoparticles and multi-walled carbon nanotubes for the detection of dichlorvos | 4 μg/L | 10–100 μg/L | [64] | |
| Reusable miRNA biosensor based on electrocatalytic properties of heterogeneous double template copper nanoclusters (CuNCs) | 8.2 fM | 25–300 fM | [65] | |
| Detection of lipopolysaccharide by aptasensor based on gold cluster | 7.94 × 10−3 amol/L | 0.01 amol/L–1 × 10−6 amol/L | [66] | |
| Carbon nanomaterials |
MIrB is used as a recognition element, and the electrode modified with -COOH functionalized MWCNT to detect microcystin-LR | 0.127 pg/mL | 1 pg/mL–100 ng/mL | [67] |
| Electrochemical biosensor using graphene oxide (GO) as a direct marker for the detection of DNA polymorphs | - | OTA:310 fM–310 pM | [68] | |
| Based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticides | 1.2 ng/mL | OPs: 3–4000 ng/mL | [69] | |
| Quantum Dots | Electrochemical biosensor for detection of miRNA-155 based on graphene quantum dots and horseradish peroxidase (HRP) | 0.14 fM | miRNA-155: 1 fM–100 pM | [70] |
| Detection of Alzheimer’s disease biomarker ApoE by electrochemical biosensor based on cadmium-selenium/zinc sulfide quantum dots | ~12.5 ng/m L | 10–200 ng/m L | [71] | |
| An electrochemical aptasensor to detect epithelial cell adhesion molecules (EpCAM) using silica nanoparticles and quantum dots | 10 amol/L | 10 amol/L–1.0 × 108 amol/L | [72] | |
| Magnetic nanoparticles | An electrochemical biosensor to detect 17-b-estradiol using magnetic molecularly imprinted polymer nanocomposites (Fe3O4-MIP) modified on the surface of screen-printed carbon electrodes (SPCE) | 20 nM | 0.05–10 μM | [73] |
| Combining magnetic nanomaterials Fe3O4NPs and HCR for simultaneous signal-guided electrochemical detection of miRNAs | miR-141:0.28 fM miR-21:0.36 fM |
1 fM–1 nM | [74] | |
| Metal-organic framework materials | Sensitivity detection of three isomers of hydroquinone, catechol, and resorcinol based on M@Pt@M-RGO electrochemical biosensor | HQ:0.015 μmol/L CT:0.032 μmol/L RS:0.133 μmol/L |
HQ:0.05–200 μmol/L CT:0.1–160 μmol/L RS:0.4–300 μmol/L |
[75] |
| An electrochemical biosensor to detect simultaneously PA and DA using HKUST-1 (Cu-BTC) coupled with graphene oxide (ERGO) | PA:0.2–160 μM DA:0.2–300 μM |
PA:0.016 μM DA:0.013 μM |
[76] | |
| An electrochemical biosensor to detect UA using CeO2-x/C/RGO nanocomposites synthesized by MOF and graphene oxide | 2.0 μmol/L | 49.8–1050.0 μmol/L | [77] |
3.2. Signal Amplification Strategies Based on Enzymes
3.3. Signal Amplification Strategies Based on Nucleic Acid Amplification Techniques
3.3.1. Signal Amplification Strategies Based on Nuclease
Nucleic acid amplification is a process for the efficient amplification of specific nucleic acid sequences and it has been often used to detect a variety of biological targets (e.g., DNA, RNA, and other small biological molecules). There are two types of target cycling principles in nucleic acid amplification, one of which involves the copying of the target chain by nucleic acid amplification, resulting in signal amplification, while the other involves a nuclease-induced target cycling-based strategy, which has been widely used in signal amplification for electrochemical biosensors. The various nuclease tool enzymes that have been often relied upon for nucleic acid amplification based on nuclease assistance consist of nucleic acid endonucleases, nucleic acid exonucleases, polymerases, and DNAzyme [59]. Double-strand-specific nucleases (DSN) are the most commonly used nucleic acid endonucleases [133]. Polymerase chain reaction (PCR), rolling circle amplification (RCA), and strand displacement amplification (SDA) are all common polymerase-dependent nucleic acid amplification reactions. PCR is a target amplification technique, and the detection principle of RCA technology involves the following. It uses a circular single-stranded DNA as a template, and under the drive of DNA polymerase, a large amount of single-stranded DNA complementary to this circular DNA template be produced, which finally amplifies a signal [59]. In addition, RCA products have been often used as carriers loaded with large amounts of electrochemical tracers to achieve electrochemical signal amplification [134,135].
Yang et al. [136] first used circular enzyme signal amplification (CESA), DSN, and 3-QD-DNA nano-complexes as cascade signal probes to achieve the ultrasensitive detection of microRNAs using dual signal amplification. Miao et al. [137] prepared an electrochemical biosensor for miRNA detection based on the catalytic reaction of double-stranded specific nuclease (DSN) and cleaved endonuclease (NEase). These researchers designed a DNA four-way linkage structure on an electrode surface, and with the assistance of a DSN cleavage reaction and enriched DNA probe that could be triggered by a target miRNA, the electrochemical material on the electrode surface was heavily consumed after the cyclic catalytic reaction. In addition, the determination of target miRNA concentration could be achieved based on the electrochemical response. Zhang et al. [138] designed an electrochemical sensor based on RCA-mediated palladium nanoparticles (PdNPs), achieving the ultrasensitive detection of microRNAs.
DSN-based signal amplification strategies have been one of the most effective ways to improve the sensitivity and specificity of electrochemical biosensor detection, and have shown good potential in the early diagnosis of diseases. However, DSN-based electrochemical biosensors still face significant challenges in the design of sensing probes. In addition, there are still many unresolved issues in terms of the standardized methods for high selectivity and sensitivity in the detection of actual clinical samples [133].
3.3.2. Signal Amplification Strategies Based on Enzyme-Free Nucleic Acids
The strand displacement reaction (SDR) is a common enzyme-free nucleic acid amplification technique, and in recent years, researchers have proposed a sticky terminal-mediated strand displacement reaction (TMSDR), which has greatly accelerated the reaction speed of SDR [59]. TMSDR amplification techniques have been widely used in biosensors due to their advantages such as being enzyme-free, their high amplification efficiency, and their controlled kinetics [59]. Both common nucleic acid amplification techniques, catalytic hairpin self-assembly (CHA), and the hybrid chain reaction (HCR) fall under the category of TMSDR. Catalytic hairpin self-assembly (CHA) achieves signal amplification by using the catalytic function of an initiator chain to release the initiator chain after forming a double-stranded structure, proceeding to the next round of catalytic hairpin self-assembly [139]. The hybrid chain reaction (HCR) consists of a probe amplification technique that uses molecular recognition and hybridization reactions to sequentially open multiple hairpin probes to achieve signal amplification through cumulative signals [140,141]. The amplification principle of HCR is shown in Figure 4, where the two sub-stable DNA hairpins (H1 and H2) complementary to each other each have a short sticky end, but are bound by the hairpin structure and can only be amplified through the addition of an initiating chain (I) and a toehold chain replacement reaction with H1, where H1 opens and a new single-stranded domain is exported to react with H2. Then, H2 opens, and a new single-stranded domain is similarly exported to react with H1. This reaction follows a chain reaction that culminates in the formation of a long dsDNA polymer [142].
Figure 4. Principle diagram of the hybridization chain reaction (HCR) amplification.
Ling et al. [143] constructed an electrochemical biosensor for efficient protein detection based on the molecular recognition between an aptamer and a target utilizing a target departure signal amplification strategy. They hybridized the auxiliary DNA1 with an aptamer, forming a double-strand, and then used the high affinity between the aptamer and target to release the auxiliary DNA1 and trigger the DNA1 circuit, eventually forming a large number of hairpin-shaped DNA3 on the electrode surface. Thus, the unfolded DNA hybridized with the DNA captured on the surface of the Pt nanoparticles would generate an electrochemical signal of H2O2 reduction for signal amplification. Cheng et al. [144] constructed an electrochemical biosensor for the efficient detection of exosomal microRNAs based on the strand displacement reaction (SDR) strategy of signal amplification of both target miRNA cycling and silver nanoparticle deposition. The detection limit of this electrochemical biosensor was shown to be 0.4 fM (S/N = 3) for miRNA-21 in exosomes [144].
CHA has the advantages of high catalytic efficiency, low background signal, and a simple and stable reaction system. The use of HCR can ensure both the sensitivity and specificity of experiments, and compared to traditional methods, HCR has been shown to be an isothermal reaction process that can be performed at room temperature, yielding a high signal-to-noise ratio [133]. Based on this signal amplification strategy, electrochemical biosensors as a detection platform for the highly sensitive detection of targets with fewer restrictions on the experimental environment provide a simple yet versatile method, with promising applications for basic research and clinical detection. However, there are still challenges, such as the ability to effectively control background signals in the analytical application of CHA, and the limitations of the HCR in terms of a low sensitivity to DNA linker formation and a low catalytic rate [145].
3.4. Signal Amplification Strategies Based on Polymers
With the development of modern technology, complex and optimized soft structures with synergistic properties have been developed to improve the sensitivity and selectivity of existing biosensors [146]. Polymers such as polyaniline (PANI), polypyrrole, and transition metal oxides have been widely used in biosensors due to their good electrical conductivity, ease of compounding with other functional materials, and low cost [60,147−149]. In addition, they can further enhance electrochemical signals by accelerating electron conductivity [91]. Transition metal sulfides, with their high electrical conductivity, large active surface area, and high catalytic activity, have also been shown to be suitable and desirable materials [150–152]. Covalent-organic frameworks (COFs) are a new polymer consisting of precisely organized organic building units with a periodic skeleton and porous crystal structure connected using covalent bonds [153]. In addition, this polymer can be used in combination with other materials (e.g., metal nanomaterials and carbon nanomaterials) to synergistically generate a large number of signals by combining the strengths of both materials, thus enabling signal amplification.
Polydopamine (PDA) is a good conductive polymer coating material that can be very easily deposited on various material surfaces [154]. Zheng et al. [153] first designed an electrochemical biosensor using polydopamine (PDA)-coated BCN as a substrate platform, methylene blue (MB) containing a MnO2-functionalized COF as a signal amplification platform and probe material, and metallic gold-platinum nanoparticles (AuPbNPs) for signal double amplification of the electrochemical biosensor to achieve the ultra-sensitive detection of PSA. These researchers used the synergistic behavior of MnO2 nanoparticles to ensure that the COF did not aggregate in this reaction, thus ensuring the stability of the composite. Molybdenum sulfide (MoS2) is a transition metal sulfide with a large specific surface area, high catalytic properties, and good optical properties. However, it is often functionalized in electrochemical biosensors due to its lack of conductivity [155–157]. Sun et al. [158] assembled an electrochemiluminescent immunosensor based on AMGM nanocomposites for the detection of PSA in serum using a so-called immunosandwich method. They prepared the MoS2/GO/o-MWNT nanocomposites with a three-dimensional flower-like structure using GR and CNTs as supporting skeletons, which were then modified with gold nanoparticles using a hot water method. The polymer amplified signal amplification by increasing antibody loading, accelerating the rate of electron transfer, and maximizing the retention of antibody activity. The ECL response of this electrochemiluminescent immunosensor was proportional to the logarithm of the PSA concentration, and had a detection limit of 0.1 pg/mL. Molecularly imprinted polymers (MIPs) and surface imprinted polymers (SIPs) have been shown to have stable physicochemical properties and have often been used in the construction of electrochemical biosensors [159].
Polymer-based electrochemical biosensors have provided great improvements in sensitivity, selectivity, stability, and the corresponding reproducibility of electrodes for the detection of a wide range of analytes [160]. Due to their having a large specific surface area and good biocompatibility, polymers can be used in combination with other materials to take advantage of both characteristics, and ultimately produce a large number of signals. Thus, polymers provide researchers with a way to prepare efficient biosensors when used in combination with other materials [159].
3.5. Signal Amplification Strategies Based on Redox Markers
In microRNA electrochemical biosensors, redox markers have typically been used to enhance the electrical signals by electrostatic aggregation on adsorbed electrode surface microRNAs or to generate heterologous double-stranded nucleic acid molecules to capture DNA/RNA for signal enhancement [133]. Methylene blue (MB), toluidine blue (TB), and hexaammonium ruthenium (RuHex) are all common redox signal indicators used in electrochemical biosensors. Tian et al. [161] constructed a simple and sensitive electrochemical biosensor for the detection of microRNA-21 by modifying an AuNP superlattice onto a glassy carbon electrode for the first time, enhancing the specific surface area and conductivity of the electrode and using the electrostatic adsorption aggregation of TB for signal amplification. Hexaammonium ruthenium (RuHex) is a positively charged electroactive complex that binds to the anion of a deoxyribonucleic acid through electrostatic interactions. Hong et al. [162] constructed an efficient electrochemical biosensor for microRNA detection using RuHex as a signal indicator and screen-printed gold electrodes (SPGEs) as a substrate. These researchers introduced two auxiliary probes self-assembled as signal amplification carriers, and when the target molecule was present, the DNA conjugate formed by the auxiliary probe self-assembly could bond to the capture probe, and then RuHex aggregated through the DNA conjugate to the working electrode, ultimately achieving signal amplification [162].
Redox molecules can bind directly and specifically to DNA without complex preparation. Therefore, direct intercalation of redox molecules can be considered a simple and rapid intercalation method. However, electrochemical biosensors based on redox labeling cannot simultaneously detect multiple substances, as the interaction of redox molecules is not specific and can be embedded on all DNA chains on an electrode surface, leading to non-specific interactions and ultimately affecting the detection results [133].
3.6. Signal Amplification Strategies Based on Cells or Tissue
Traditionally, tissue sensors are a class of biosensors that use animal and plant tissue-thin layer slices as receptors for signal amplification by utilizing the catalytic action of enzymes in natural tissues. Microbial biosensors are composed of molecular recognition elements (microbial sensitive membranes) and signal transducers. A microbial sensitive membrane can be fabricated by applying immobilization techniques to immobilize microorganisms onto a carrier without damaging their function. The principle of operation involves using respiration or metabolism for signal amplification purposes. In recent years, studies have shown that cells or tissues can be used directly as sensitive elements to construct corresponding electrochemical biosensors for the detection of targets and for signal amplification [163].
Cells can sense small changes in environmental signals through receptor–ligand interactions, and these signals can be amplified through a cellular system of signaling cascades, which can cause changes in membrane potential and depolarization by regulating ion channel switches; thus converting signals into neural signals that can be transmitted to brain nerve centers to produce rapid responses [164]. In addition, these signals can be transmitted to the nucleus via cellular signal amplification pathways, and then signals such as metabolism are sent throughout the body by the regulation of gene expression, thus allowing the system to respond to environmental factors [165]. One of the primary components of this process is the G-protein cascade amplification system, which is coupled with multiple receptors to sense and amplify cellular signals [164]. To explore whether GPCRs can trigger G-protein signal amplification in tissues or cells of different species, Xu et al. [164] self-assembled bombykol receptors onto the cell membranes of taste bud tissues of different species. Eventually, a novel bombykol receptor sensor was established to detect G-protein signal amplification. The results showed that the G-protein signal cascade amplification system was universal in the GPCR of different tissues and species. Among them, the bombykol receptor sensor self-assembled with the cattle taste tissue, which was the most sensitive toward bombykol, with a lower limit of detection of about 1 × 10−19 mol/L.
Wei et al. [166] constructed an electrochemical taste biosensor by simulating the neurotransmission mechanism of taste, using porcine taste bud tissue as the sensitive element to measure the effects of bitter taste receptors interacting with sucrose octaacetate, benzoate, and quercetin. The results showed that this sensor could be used to characterize cellular or tissue receptor–ligand interactions and its cellular signal cascade amplification. In addition, the signal amplification of the taste bud cells was more than ten orders of magnitude. Resveratrol is a polyphenol phytoalexin found in a variety of plants that has been shown to have a wide range of health benefits in animal studies [167–170] and beneficial biopharmacological activity against cancer in humans [171]. However, a limited number of studies have evaluated its interactions with cell surface receptors. Ren et al. [172] constructed a sandwich rat small intestinal tissue sensor (RSIT sensor) based on rat small intestinal tissue cells as the sensitive elements and effectors to detect the corresponding currents of different concentrations of resveratrol for receptor stimulation by electrochemical means, and compared the response values of this electrochemical biosensor and the bare electrodes prepared in this study with those of resveratrol. The results showed that the lower limit of detection was 1 × 10−13 mol/L, and the intracellular signaling system significantly amplified the response values of the small intestinal cells to resveratrol, by approximately 100 times.
Electrochemical biosensors based on cells and tissues have shown good stability and accuracy and have broad application prospects for pheromone detection, sex pheromone detection, and receptor structure and function. However, further research is needed to determine whether the immobilization of cells affects receptor–ligand interactions.
3.7. Signal Amplification Strategies Based on Microfluidics
Microfluidics refers to the science and technology of precisely controlling and manipulating micro and nanofluids with high precision and reproducibility in the micro and nanoscale space [173,174]. Microfluidic chips, also known as lab-on-a-chip (LOC), can integrate the basic functional units involved in biological and chemical experiments, such as mixing, reaction, separation and detection, on a single chip with a high degree of integration [175]. In addition, the microfluidic chip is a critical part of the biosensor for microfluidic control technology, and its structure is shown in Figure 5. It mainly consists of three parts: a micro-mixer with staggered asymmetric herringbone recesses, a serpentine culture channel, and a separation chamber[]. As a key component of the microfluidic chip, the micromixer often has a great impact on the sensitivity of the microfluidic biosensor. The top or bottom of the microchannel has been shown to promote lateral flow, increase local vorticity and achieve effective mixing178].
Figure 5. The structure of microfluidic chip.
In recent years, there has been an increasing awareness of health and a growing need for the prevention and diagnosis of several diseases. The combination of microfluidics and advanced biosensing technologies has given rise to many excellent miniaturized analytical platforms that enable the precise control of micro- and nano-fluids and the integration of various types of bio arrays on a miniaturized platform [174]. Microfluidics, due to its rapid development, has gradually become the main means of implementation for POCT diagnostics. Electrochemical biosensors based on microfluidic control technology have been frequently applied for the monitoring of cells and cellular components, proteins and small molecules, etc. [179]. Hazal Kutluk et al. [180] constructed a low-cost microfluidic biosensor for the electrochemical measurement of miRNA-197 (a tumor biomarker candidate) in undiluted human serum samples, which required a very low sample volume (580 nl) and short detection time. They developed two different formats (sandwich assay and competition assay) for miRNA determination and compared them in terms of sensitivity, accuracy and simplicity. The results showed that the sandwich assay had a superior performance in terms of sensitivity and selectivity with a minimum detection limit of 1.28 nM. Cardiac troponin I (cTnI) is an attractive biomarker for acute myocardial infarction (AMI). Yang L et al. [181] developed a sensitive portable microfluidic electrochemical array device (μFED) for the immunoassay of trace human cardiac troponin I (cTnI). They used an alkaline phosphatase (AP) label for signal amplification and improved the performance of the μFED by eliminating the shielding effect of the microelectrode array (MEA) integrated with the μFED. The results showed that the detection time for cTnI was only 15 min and the lower limit of detection was 5 pg/mL (S/N = 3).
In summary, microfluidic integrated biosensor devices have many advantages, such as low reagent consumption, short reaction time, automated sample preparation, high throughput analysis, high detection accuracy and portability, and low cost, and are often applied to the detection of various substances [182,]. However, most of the current microfluidic products are limited to the laboratory stage of scientific research, and there are few mature products on the market. Moreover, the application of microfluidics is limited by the ultra-high precision processing requirements, the challenge of precise control of micro- and nano-sized liquids, and the problem of how to achieve rapid mass production at a low cost. In recent years, the introduction of various functional materials has facilitated the development of multifunctional microfluidic control chips, which have broadened the application areas of this type of biosensor and are expected to shine in the biomedical field [174].
3.8. Signal Amplification Strategy for the Combination of Multiple Materials
In recent years, to achieve the amplification of electrical signals, researchers have moved away from using a single material and instead have designed signal amplification strategies based on the combined use of multiple materials, each of which has its advantages in various detection methods. These include metal nanomaterials for their high electrical conductivity and electron transfer capability, carbon nanomaterials for their large specific surface area, high electrical conductivity, and physical-chemical stability, and enzymes for their high catalytic properties and specificity. For example, Zhang et al. constructed a highly sensitive electrochemical biosensor for microRNA detection based on three signal amplification strategies, namely double-stranded specific nuclease (DSN)-assisted target cycling, gold nanoparticles (AuNPs), and HRP enzyme-catalyzed reactions. These researchers combined excellent enzymatic activity with a large specific surface area and better controlled the surface properties of gold nanoparticles [90,185], as well as using the strong differentiation ability of DSNs to achieve signal amplification with high sensitivity and selectivity. Chen [186] et al. also developed an electrochemical DNA biosensor combining three signal amplification techniques using the rolling circle amplification (RCA) shear recovery of target DNA, gold nanoparticle labeling, and multiple probes for the detection of MON89788 in soybean transgenic gene sequences. This electrochemical biosensor has a detection limit of 4.5 × 10−17 mol/L, and it has been often used for targeted gene sequence analysis because of its high sensitivity. Ferrocene (FC) has often been used as a signaling marker molecule due to its active reversible redox electron and excellent electrochemical signaling ability. Zhang et al. [187] used synthesized bis (ferrocene-labeled) hairpin DNA (placed at both ends of hairpin DNA) and integrated it into an electrochemical biosensor together with catalytic hairpin assembly (CHA) to achieve the ultra-sensitive detection of a microRNA, using the dual signal amplification of both. The synthesized double ferrocene produced two ferrocenes, which enhanced the signal response through their superposition effect, and this electrochemical biosensor had a minimum detection limit of 0.1 fp. Table 2 lists some applications of electrochemical biosensors based on the above signal amplification in practical detection.
Signal amplification strategies based on other materials.
| Strategies | Examples | Limit of detections | Linearity ranges | Ref. |
| Enzyme | Electrochemical immunosensor based on DT-diaphorase (DT-D) as oxidoreductase labeling and 4-nitroso-1-naphthol (4-NO-1-N) as reaction substrate | PTH:2 pg/mL | 2 pg/mL-1μg/mL | [125] |
| Electrochemical biosensor based on the display of tyrosinase on the surface of Escherichia coli cells for the detection of Bisphenol A | 0.01nm | BPA:0.01nm-100nm | [126] | |
| Nucleic acid amplification | An electrochemical biosensor based on cyclic enzyme signal amplification (CESA) with DSN and 3-QD-DNA nanocomposites as cascade signal probes for hypersensitive detection of microRNA | 1.2amol/L | 5amol/L-5fmol/L | [136] |
| An electrochemical biosensor using double-stranded specific nuclease (DSN) and cleavage endonuclease (NEase) catalyzed reactions to detect miRNA | 3aM | 10aM-10fM | [137] | |
| Ultra-sensitive detection of microRNA by an electrochemical biosensor based on RCA-mediated palladium nanoparticles (PdNPs) | 8.6amol/L | 50amol/L-100fmol/L | [138] | |
| Protein detection by electrochemical biosensors based on molecular recognition between aptamer and target | 0.17 pM | 0.5pM-300nM | [143] | |
| Efficient detection of exosomal microRNAs by strand displacement reaction (SDR) based electrochemical biosensor | 0.4fM | miRNA-21:1fM-200 pM | [144] | |
| Polymers | Electrochemical biosensor based on methylene blue (MB) containing MnO2-functionalized COF, and metallic gold-platinum nanoparticles (AuPbNPs) for ultra-sensitive detection of PSA | 16.7 fg mL-1 | 0.00005-10 ng mL-1 | [153] |
| Electrochemiluminescent immunosensor based on AMGMs nanocomposites for the detection of PSA in serum | 0.1pg/ml | PSA:0.1pg/mL- 50ng/mL | [158] | |
| Redoxmarkers | An electrochemical biosensor to detect microRNA-21 using toluidine blue (TB) electrostatic adsorption aggregation signal amplification | 78amol/L | 100amol/L-1nmol/L | [161] |
| An electrochemical biosensor based on RuHex and screen-printed gold electrodes (SPGEs) to detect microRNA | 100amol/L | 100amol/L-100pmol/L | [162] | |
| Cell or tissue | The RSIT sensor by using rat small intestine tissue cells as a sensitive element and effector to detect resveratrol | 1 × 10-13mol/L | - | [172] |
| Cell membrane biosensor with hTRPV1 immobilized directly on the HEK293T cell membrane to detect spicy substances | - | - | [188] |
- Summary and Outlook
In recent years, with the continuous development of science and technology, such as materials, electronics, signal processing technique and gene editing technologies, signal amplification strategies based on electrochemical biosensors have made great progress and have become widely used. In this review, we researched and presented the progress on olfactory and taste electrochemical signal amplification strategies. Taste receptors have very important neurological, physiological, immunological, and endocrine functions in the presence of taste components [34]. In addition, we summarized various methods for enhancing electrochemical signals. Gold nanomaterials, carbon nanomaterial QDs, and other nanomaterials and enzymes commonly used as electrode materials, redox tracers catalytic markers, and carriers of signal elements [80], as well as signal amplification using nucleic acid amplification techniques have played an important role in enhancing sensitivity and improving the performance of electrochemical biosensors.
Electrochemical biosensors based on the above-mentioned signal amplification strategies will undoubtedly offer great potential for practical sample detection and olfactory taste determination in humans and animals. Currently, recent achievements in biotechnology have led to the possibility of modulating the affinity of bioreceptors for selected VOCs by gene editing techniques to design highly selective biosensors [8]. Furthermore, OR-based biosensors have great potential to become bioelectronic snuff systems for detecting VOCs in areas including food safety, environmental and industrial monitoring, clinical diagnostics, agricultural diseases, and drug development [31]. However, current electrochemical sensors still have some shortcomings. For example, the instability of their characteristics and final analytical performance between sensors in practical applications may lead to experimental failures [189]. Therefore, the development of electrochemical biosensors with high accuracy and stability that are suitable for general promotion and use still requires continuous effort to promote the development of electrochemical biosensors toward functional diversification, miniaturization, and integration, offering more extensive application prospects for disease diagnosis, genetic testing and other fields [127]. We believe that competitive research and large commercial opportunities in the field of biosensors will lead to exciting new developments in the near future.
This entry is adapted from the peer-reviewed paper 10.3390/bios12080566
