Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Columnar cell lesions (CCLs) of the breast comprise a spectrum of morphologic alterations of the terminal duct lobular unit involving variably dilated and enlarged acini lined by columnar epithelial cells. The World Health Organization classifies CCLs without atypia as columnar cell change (CCC) and columnar cell hyperplasia (CCH), whereas flat epithelial atypia (FEA) is a unifying term encompassing both CCC and CCH with cytologic atypia.
- breast
- columnar cell lesions
- precursor
- low-grade neoplasia
- carcinogenesis
1. Introduction
Columnar cell lesions (CCLs) of the breast comprise a spectrum of morphologic alterations of the terminal duct lobular unit (TDLU) involving variably dilated and enlarged acini lined by columnar epithelial cells. While CCLs have historically been described using a variety of terms, the World Health Organization currently classifies CCLs without atypia as columnar cell change (CCC) and columnar cell hyperplasia (CCH), whereas flat epithelial atypia (FEA) exhibits low-grade (monomorphic) cytologic atypia and may be used as a unifying term encompassing both CCC and CCH with atypia [1].
In part due to the implementation of widespread screening mammography, CCLs have been increasingly recognized in stereotactic core needle biopsies (CNB) performed for the assessment of calcifications [2][3][4][5]. CCLs have been postulated to represent the earliest non-obligate precursor of low-grade invasive breast carcinomas as they share molecular alterations and often coexist with entities in the low-grade breast neoplasia pathway, including atypical ductal hyperplasia (ADH), lobular neoplasia (LN), low-grade ductal carcinoma in situ (DCIS) and tubular carcinoma [6][7][8][9][10][11][12][13][14][15][16][17][18][19][20]. Despite this association, however, the risk of progression of CCLs to invasive breast carcinoma is low and may not exceed that of concurrent proliferative lesions. As the reported rates of pure CCL/FEA when identified as the most advanced high-risk lesion on CNB vary widely, the management of FEA diagnosed on CNB remains controversial.
2. Historical Overview and Terminology
CCLs have long been recognized by pathologists but have been previously described using a wide assortment of names, including blunt duct adenosis [21], columnar alteration of lobules [6], columnar cell alterations with atypical snouts [6], atypical cystic lobules [7], atypical lobule of type A [22], hyperplastic enlarged lobular unit [23], unfolded lobules [24], monomorphous-type clinging carcinoma [25], and flat epithelial atypia without intraluminal proliferation, originally designated as ductal intraepithelial neoplasia 1b (DIN1b) [26] and later DIN1a [27]. CCLs with more complex architectural patterns such as micropapillary formations, rigid cellular bridges, bars and arcades or sieve-like fenestrations have also been previously categorized as CCH with moderate or severe atypia [16].
In 2003, Drs. S. Schnitt and A. Vincent-Salomon grouped CCLs into two categories-CCC and CCH, both of which should lack significant cytologic atypia. In contrast, the presence of high-grade nuclear features, even if present in a single cell layer, merits the designation of high-grade DCIS, while complex architectural patterns are best classified as ADH or low-grade DCIS depending on their extent [28]. In 2005, this classification was expanded into six groups by Simpson et al.: (1) CCC, (2) CCH, (3) CCH with architectural atypia, (4) CCH with cytologic atypia, (5) CCH with cytologic and architectural atypia, and (6) CCC with cytologic atypia. The authors noted difficulties in interobserver reproducibility encountered due to limitations in assessing mild nuclear pleomorphism [29]. In 2007, while investigating possible precursor lesions less than ADH or DCIS, Goldstein et al. defined monomorphic epithelial proliferations (MEPs) as a slightly overcrowded, predominantly single layer of monomorphic, luminal epithelial cells involving TDLUs in an overgrowth extension pattern [30]. MEPs tended to retain the architecture of the TDLU but could also expand the acini in an unfolded lobule-like pattern without associated complex architectural features. The cytomorphologic features of MEPs ranged from innocuous to slightly atypical and included three patterns: (1) columnar cells with uniform ovoid to elongated nuclei, (2) cuboidal cells with mildly enlarged round to oval nuclei, and (3) cuboidal to columnar cells with scant cytoplasm and round to slightly ellipsoid nuclei. The MEP entity included any lesion that was monomorphic and slightly hypercellular, regardless of the cytologic features, and was not restricted to lesions termed by others as CCLs or FEA. MEP lesions were significantly more frequent near initial breast resection margins in patients with clonally related local breast cancer recurrences than in patients with clonally distinct recurrences or no recurrences. Furthermore, some MEPs in clonal and distinct local recurrences shared allelic imbalance microsatellite markers with their respective initial carcinomas. Thus, MPEs appear to form the pool of partially transformed precursor lesions less than ADH or DCIS, similar to CCLs.
Blunt duct adenosis (BDA) was first described by Foote and Stewart [21], and has previously been considered a synonym of CCL without atypia [31][32]. Other authors, however, describe morphologic differences between BDA and CCL, including flattened branching configurations, irregular ducts, conspicuous myoepithelial cells with clear cytoplasm and slightly expanded, fibrotic and cellular intralobular stroma [33][34]. In the 2012 edition of the WHO classification of tumors of the breast, BDA is considered synonymous with CCC/CCH and is distinguished from FEA by the lack of cytologic atypia [35]; however, in the 2019 edition BDA is mentioned as “not recommended terminology” for CCLs [1]. De Boer et al. sought to assess molecular characteristics of low-grade breast neoplasia (whole-arm loss of chromosome 16q) using multiplex ligation-dependent probe amplification in a cohort of BDA and CCLs with and without atypia [36]. 16q loss was common in CCLs with and without atypia but was absent in BDA. In a subsequent publication, the authors stated that given the lack of 16q loss together with recognizable architectural and cytonuclear features, BDA is not a true precursor in the low-grade neoplasia family and should be considered as a separate entity from CCLs [37]. Follow-up data was also recommended to assess the reproducibility of the histologic diagnoses of BDA versus CCL and to determine if the risk of progression to breast cancer of BDA is that of other benign lesions.
The current 5th edition of the WHO classification of tumors of the breast from 2019 classifies CCLs without atypia as CCC and CCH. FEA is the recommended term for CCLs with atypia, although acceptable terminology also includes CCC with atypia and CCH with atypia [1].
3. Radiologic Findings
CCLs are most frequently identified in CNBs performed for mammographically detected calcifications. While the majority of CCLs manifest as grouped amorphous calcifications, fine pleomorphic or punctate calcifications can also be observed [2][3][6][8][9][10][32][38][39][40][41][42][43][44][45][46][47][48][49]. The calcifications associated with CCLs may be indistinguishable from other causes of suspicious calcifications such as ADH or DCIS, necessitating biopsy or excision for diagnosis [2]. Less often FEA may appear as a mammographically detected mass, architectural distortion or asymmetry [10][41][42][43][44][46][47][49][50]. Some CCLs may not be detected due to their small size and lack of associated calcifications or other radiologic abnormalities.
Sonographically, CCLs with and without atypia may present as cystic or solid lesions as well as a mass with irregular, microlobulated or indistinct borders and hypoechoic or complex echotexture [2][38][39][43][45][46]. There are no reported specific mammographic or ultrasound features which aid in the distinction of patients with CCLs with or without atypia [2][45].
Although there is a dearth of literature with regards to magnetic resonance imaging (MRI) features in CCLs, findings described include non-mass enhancement (NME) [38][51] and irregular clumped enhancement [46]. Santucci et al. describe a series of 139 patients with histologic borderline (B3) lesions, of which 31 (14.39%) were diagnosed as FEA [47]. Six patients in this cohort had MRI investigations, including five (83%) with post-contrast mass-like enhancement and one (17%) with segmental enhancement. The borders of the masses were described as regular, lobulated, irregular and with blurred edges. Kinetic curves were evenly split between types I, II and III. CCLs diagnosed purely as MRI findings with no mammographic or ultrasound correlates appear exceptionally rare [52][53][54][55][56].
Approximately 40% of women in breast screening programs have mammographically dense breasts. Percent mammographic density (PMD) of the breast reflects variations in the number of non-epithelial and epithelial cells, and collagen. Extensive PMD or elevated mammographic density (MBD) is associated with an increased risk of developing invasive carcinoma [57]. A possible association between CCLs and breast tissue composition was first reported in 2009 [58]. In a cohort of 236 randomly selected tissue samples obtained by bilateral subcutaneous mastectomy from a forensic autopsy series, CCLs were identified in 40 (17%) cases. The presence of CCL was associated with measures of breast tissue composition, such as high Faxitron Wolfe Density, high density estimated by percentage non-adipose tissue area, high percentage collagen, and high percentage glandular area. In a more recent study of 3400 women, after adjusting for age and body mass index, there was a positive association between CCH/FEA and high MBD (OR 1.3, 95% CI, 1.0–1.6) [59].
4. Histologic Features
The simplest form of CCL is CCC which is characterized by variably dilated acini with relatively smooth contours lined by one to two layers of columnar epithelial cells (Figure 1a,c). The lesional cells of CCC exhibit ovoid to elongated nuclei that are oriented perpendicular to the basement membrane in a regular fashion and contain evenly dispersed chromatin without conspicuous nucleoli (Figure 1b,d–f). Apical cytoplasmic blebs or snouts may be present at the luminal surface (Figure 1d–f), and mitotic figures are rarely encountered.
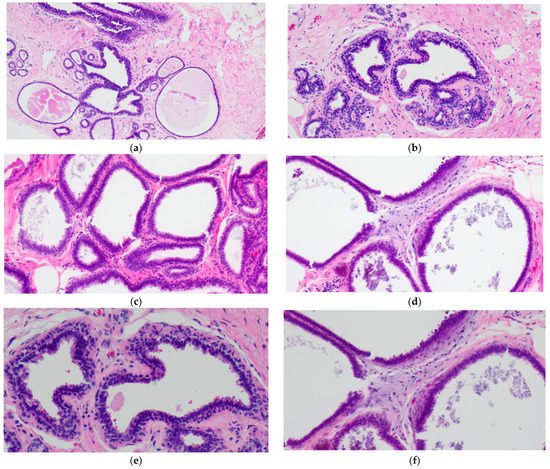
Figure 1. Columnar cell change (CCC) showing variably sized and shaped (a,c) acini lined by one to two layers of columnar-shaped epithelial cells with uniform, ovoid to elongated nuclei oriented perpendicular to the basement membrane; apical cytoplasmic snouts are seen at the luminal surface and an outer layer of myoepithelial cells is evident (b,d–f). There are luminal secretions (a,c,d) and calcifications (d,f).
CCH is composed of variably dilated acini lined by columnar cells with cytologic features similar to CCC but with cellular stratification of more than two cell layers (Figure 2a,c,e,f). Crowding or overlapping of the nuclei may give the appearance of nuclear hyperchromasia, and the proliferating cells may form small mounds, tufts or short micropapillations (Figure 2b,d). Both CCC and CCH can be associated with luminal secretions (Figure 1a,c,d and Figure 2a,b,e) and calcifications (Figure 1d,f and Figure 2c,d,f).
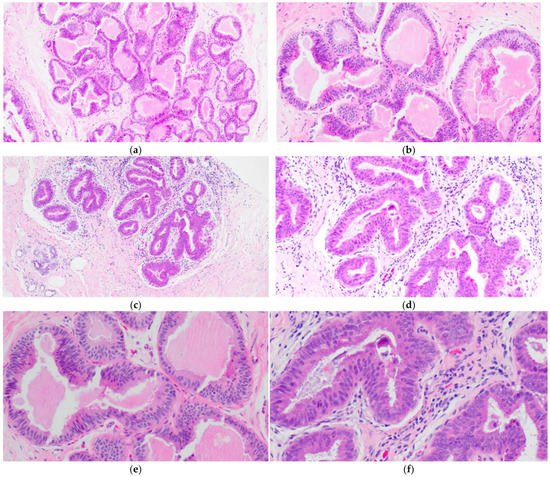
Figure 2. Columnar cell hyperplasia (CCH) showing variably dilated acini lined by more than two layers of columnar-shaped cells with (a,c,e,f) with nuclear crowding and overlapping, and formation of small tufts (b,d). There are luminal secretions (a,b,e) and calcifications (c,d,f).
FEA now encompasses the term for CCLs with low-grade (monomorphic) cytologic atypia resembling the nuclei of low-grade DCIS. FEA has architectural features of CCC or CCH (Figure 3a,c), but with superimposed cytologic atypia characterized by the presence of rounded or ovoid nuclei with loss of polarity, mildly increased nuclear to cytoplasmic ratio and variably prominent nucleoli (Figure 3b,d–f). Some cases of CCL may lack such classic morphologic features but the presence focal or patchy cytologic atypia may be sufficient to warrant the diagnosis of CCL with atypia. The latter is indeed a term preferred over FEA by some pathologists. Lesions with columnar cells but more complex architectural patterns such as rigid cellular bridges, micropapillations or arcades should be classified as ADH or low-grade DCIS (Figure 4a–f). Similarly, high-grade cytologic atypia with nuclear pleomorphism of the type seen in high-grade DCIS is not a feature of lesions included in the CCL category; such lesions should be classified as high-grade DCIS.
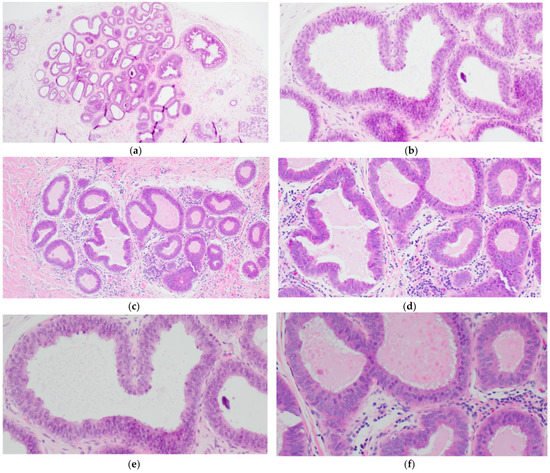
Figure 3. Flat epithelial atypia (FEA) composed of variably dilated acini (a,c) with calcifications (a,b) and low-grade cytologic atypia characterized by the presence of rounded or ovoid nuclei with loss of polarity, mildly increased nuclear to cytoplasmic ratio and prominent nucleoli in some cells (b,d–f).
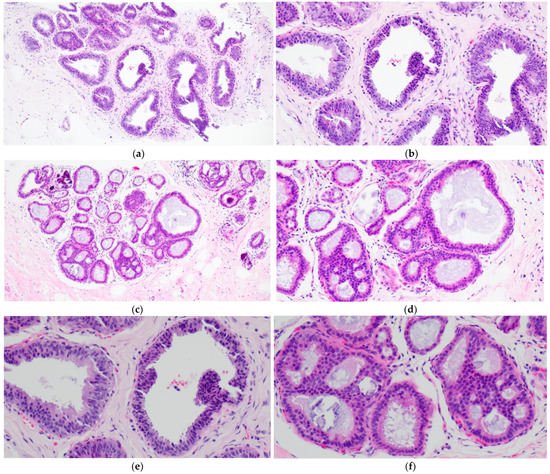
Figure 4. Columnar cell lesion (CCL) with foci of architectural complexity comprising focal formation of a micropapillary structure (a,b,e) and cribriforming with rigid cellular bridges (c,d,f) associated with low-grade cytologic atypia, consistent with atypical ductal hyperplasia.
Several authors have examined morphometric nuclear features in order to aid in the distinction of FEA from CCC. Using whole slide imaging and ImageJ software Katayama et al. compared morphologic parameters of 12 cases of CCC and FEA [60]. While cells in CCC had greater mean maximum Feret’s diameter (defined as the measure of an object size along a specified direction), major axis length and perimeter, cells in FEA had larger mean minimum Feret’s diameter, minor axis lengths and nuclear area. The nuclei of FEA also showed a significantly rounder shape as compared to nuclei of CCC. Similarly, in a cohort of 22 FEAs and 13 CCLs without atypia, Yamashita et al. used ellipticity as a nuclear parameter and also found that FEA has significantly rounder nuclei as compared to CCC/CCH [61]. These findings are in contrast to Lim et al. who reported that the Feret’s diameter, nuclear area and perimeter of FEA were significantly greater than in CCC whereas no difference was observed in circularity [62]. Overall, while nuclear morphology may distinguish FEA from CCC, further validation of morphometric features is required in order to determine which parameter is the optimal objective reference for diagnosis or in order to consider training artificial intelligence (AI)-based diagnostic algorithms.
Another point that merits consideration when discussing the histologic features of CCLs is their diagnostic reproducibility. Samples et al. assessed interobserver agreement of 29–30 pathologists for the diagnosis of six reference cases of FEA [63]. The rate of agreement with the reference diagnosis ranged from 17% to 52% with the reference panel pathologists demonstrating substantial variability in their interpretation of FEA. Tan et al. assessed the interobserver variability in a series of digitized images of CCLs presented to seven staff pathologists, and reported an intraobserver agreement of fair to substantial for the spectrum of CCLs (k = 0.334–0.669) [64]. The lowest number of complete agreements was achieved in the lesions characterized as CCC with cytologic atypia. When comparing the interobserver variability in the diagnoses of CCLs between general pathologists and a single pathologist with expertise in breast pathology, Gomes examined 610 breast specimens which were reviewed as part of a request for an external opinion (4.1% of these requests were generated by pathologists) [65]. There was weak diagnostic agreement between the original report and later review for CCC (k = 0.38); however, the agreement was moderate (k = 0.47) between the diagnoses of FEA.
Following training tutorials and with well-defined criteria for FEA, O’Malley et al. found an overall agreement of 91.8% for 30 cases of CCLs including complete agreement among all eight pathologists in 24 (80%) cases (k = 0.83) [66]. Darvishian’s group similarly found a kappa value of 0.85 when evaluating the reproducibility of FEA on CNB following a tutorial [67]. Haupt et al. analyzed the diagnostic agreement of CCL cases between residents and fellows before and after a tutorial on the diagnostic criteria of CCLs [68]. Before the tutorial the diagnostic agreement of FEA was weak (k = 0.39); however, after training there was a statistically significant increase in the ability to recognize FEA (k = 0.60). Therefore, this evidence suggests that interobserver variability may be improved following educational tutorials and using well-defined diagnostic criteria, although uniform classification of CCLs, especially FEA, may be challenging amongst pathologists in routine practice.
5. Cytologic Features
With the widespread use of CNB sampling, the cytologic features of CCLs are less relevant in the day-to-day work of practicing pathologists. Nevertheless, they have been described in several small cohorts. In a series of 20 fine needle aspirations (FNAs) with subsequent histologic diagnoses of CCLs, Saqi et al. described three-dimensional groups with overlapping cells, palisading columnar cells and occasional apocrine snouts [69]. Although 18 of 20 FNAs were described as atypical, only five of the cases demonstrated cytologic atypia on subsequent CNB. In another series of 10 cases of CCLs sampled by FNA followed by surgical excision, Jensen et al. describe sheets and papillary clusters of cells with well-delineated cell borders, round nuclei and finely granular cytoplasm with atypia ranging from mild to suspicious for malignancy [70]. Both studies note that due to substantial overlap with the cytologic features of other entities such as papillary lesions, fibroadenomas, malignant breast neoplasms and radiation changes, CCLs cannot be reliably diagnosed by FNA.
6. Immunohistochemical Markers
The immunohistochemical features of CCLs have been investigated in order to examine their relationship to other breast lesions and explore their etiology. Similar to other low-grade clonal proliferations such as ADH and low-grade DCIS, CCLs are usually diffusely positive for estrogen and progesterone receptors [7][11][23][24][29][71][72], cytokeratin 19 [7][11][29], Bcl-2 [73], and cyclin D1 [7]. High molecular weight cytokeratins (CK5/6, CK14) are largely negative [29][74] as is HER2 [75][76]. P53 expression has been found to parallel that of adjacent carcinoma [75].
Noel et al. investigated the proliferative rate in CCLs and found that the Ki-67 index was low (<1%) in CCC and CCH without atypia [77]. While the proliferative index was significantly higher (mean 8.2%) in FEA, it was lower than that of intermediate to high-grade DCIS (mean 25.4%). Paradoxically, the Ki-67 proliferative index in CCC and CCH without atypia was lower than in the adjacent TDLU (mean 2.4%), a finding postulated to be due to the fact that TDLUs are less sensitive to the effects of the menstrual cycle, age, oral contraceptive of hormonal replacement therapy, or because CCC without atypia represents only a simple transformation from cuboidal to tall columnar epithelium without modification of proliferative properties.
When examining a series of breast biopsies performed for mammographically detected calcifications, Fraser et al. described a spectrum of lesions in the TDLU characterized by columnar epithelial cells with prominent apical snouts, intraluminal secretions, varying degrees of cytologic and architectural atypia and associated calcifications which they termed “columnar alteration with prominent apical snouts and secretions (CAPSS)” [6]. Using CAPSS terminology, Dessauvagie et al. found Ki-67 immunostaining (9.16 ± 4.07%) increased as compared to normal breast (1.9 ± 0.67%) but lower than in situ and invasive lesions [72]. Similarly, Lee et al. found the Ki-67 proliferative index to be higher in CCLs designated “hyperplastic enlarged lobular unit (HELU)” (6.3 ± 0.47%) than in normal TDLUs (2.0 ± 0.42%), although there was no stratification based on the presence or absence of accompanying cytologic or architectural atypia [23]. Tomasino and colleagues investigated Ki-67 expression in biopsies with FEA/CCLs with “mild” atypia (FEA/CCHAm), “high” atypia (FEA/CCHAh) and normal breast tissue. The percentage of positive nuclei in normal tissue controls was 2.2 ± 0.50, 1.8 ± 0.60 in the CCHs, 3.0 ± 0.50 in the FEA/CCHAm, and 10.5 ± 1.40 in the FEA/CCHAh [78].
This entry is adapted from the peer-reviewed paper 10.3390/curroncol29080447
References
- Hoon Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. (Eds.) World Health Organization Classification of Tumours of the Breast, 5th ed.; IARC Press: Lyon, France, 2019.
- Kim, M.J.; Kim, E.-K.; Oh, K.K.; Park, B.-W.; Kim, H. Columnar Cell Lesions of the Breast: Mammographic and US Features. Eur. J. Radiol. 2006, 60, 264–269.
- Verschuur-Maes, A.H.J.; van Gils, C.H.; van den Bosch, M.A.A.J.; De Bruin, P.C.; van Diest, P.J. Digital Mammography: More Microcalcifications, More Columnar Cell Lesions without Atypia. Mod. Pathol. 2011, 24, 1191–1197.
- Luiten, J.D.; Korte, B.; Voogd, A.C.; Vreuls, W.; Luiten, E.J.T.; Strobbe, L.J.; Rutten, M.J.C.M.; Plaisier, M.L.; Lohle, P.N.; Hooijen, M.J.H.; et al. Trends in Frequency and Outcome of High-Risk Breast Lesions at Core Needle Biopsy in Women Recalled at Biennial Screening Mammography, a Multiinstitutional Study. Int. J. Cancer 2019, 145, 2720–2727.
- Said, S.M.; Visscher, D.W.; Nassar, A.; Frank, R.D.; Vierkant, R.A.; Frost, M.H.; Ghosh, K.; Radisky, D.C.; Hartmann, L.C.; Degnim, A.C. Flat Epithelial Atypia and Risk of Breast Cancer: A Mayo Cohort Study. Cancer 2015, 121, 1548–1555.
- Fraser, J.L.; Raza, S.; Chorny, K.; Connolly, J.L.; Schnitt, S.J. Columnar Alteration with Prominent Apical Snouts and Secretions: A Spectrum of Changes Frequently Present in Breast Biopsies Performed for Microcalcifications. Am. J. Surg. Pathol. 1998, 22, 1521–1527.
- Oyama, T.; Maluf, H.; Koerner, F. Atypical Cystic Lobules: An Early Stage in the Formation of Low-Grade Ductal Carcinoma in Situ. Virchows Arch. 1999, 435, 413–421.
- Guerra-Wallace, M.M.; Christensen, W.N.; White, R.L. A Retrospective Study of Columnar Alteration with Prominent Apical Snouts and Secretions and the Association with Cancer. Am. J. Surg. 2004, 188, 395–398.
- Bianchi, S.; Bendinelli, B.; Castellano, I.; Piubello, Q.; Renne, G.; Cattani, M.G.; Di Stefano, D.; Carrillo, G.; Laurino, L.; Bersiga, A.; et al. Morphological Parameters of Flat Epithelial Atypia (FEA) in Stereotactic Vacuum-Assisted Needle Core Biopsies Do Not Predict the Presence of Malignancy on Subsequent Surgical Excision. Virchows Arch. 2012, 461, 405–417.
- Khoumais, N.A.; Scaranelo, A.M.; Moshonov, H.; Kulkarni, S.R.; Miller, N.; McCready, D.R.; Youngson, B.J.; Crystal, P.; Done, S.J. Incidence of Breast Cancer in Patients with Pure Flat Epithelial Atypia Diagnosed at Core-Needle Biopsy of the Breast. Ann. Surg. Oncol. 2013, 20, 133–138.
- Oyama, T.; Iijima, K.; Takei, H.; Horiguchi, J.; Iino, Y.; Nakajima, T.; Koerner, F. Atypical Cystic Lobule of the Breast: An Early Stage of Low-Grade Ductal Carcinoma in-Situ. Breast Cancer 2000, 7, 326–331.
- Abdel-Fatah, T.M.A.; Powe, D.G.; Hodi, Z.; Lee, A.H.S.; Reis-Filho, J.S.; Ellis, I.O. High Frequency of Coexistence of Columnar Cell Lesions, Lobular Neoplasia, and Low Grade Ductal Carcinoma in Situ with Invasive Tubular Carcinoma and Invasive Lobular Carcinoma. Am. J. Surg. Pathol. 2007, 31, 417–426.
- Gopalan, A.; Hoda, S.A. Columnar Cell Hyperplasia and Lobular Carcinoma in Situ Coexisting in the Same Duct. Breast J. 2005, 11, 210.
- Brandt, S.M.; Young, G.Q.; Hoda, S.A. The “Rosen Triad”: Tubular Carcinoma, Lobular Carcinoma in Situ, and Columnar Cell Lesions. Adv. Anat. Pathol. 2008, 15, 140–146.
- Sahoo, S.; Recant, W.M. Triad of Columnar Cell Alteration, Lobular Carcinoma in Situ, and Tubular Carcinoma of the Breast. Breast J. 2005, 11, 140–142.
- Rosen, P.P. Columnar Cell Hyperplasia Is Associated with Lobular Carcinoma in Situ and Tubular Carcinoma. Am. J. Surg. Pathol. 1999, 23, 1561.
- Leibl, S.; Regitnig, P.; Moinfar, F. Flat Epithelial Atypia (DIN 1a, Atypical Columnar Change): An Underdiagnosed Entity Very Frequently Coexisting with Lobular Neoplasia. Histopathology 2007, 50, 859–865.
- Gomes, D.S.; Balabram, D.; Porto, S.S.; Gobbi, H. Lobular Neoplasia: Frequency and Association with Other Breast Lesions. Diagn. Pathol. 2011, 6, 74.
- Brogi, E.; Oyama, T.; Koerner, F.C. Atypical Cystic Lobules in Patients with Lobular Neoplasia. Int. J. Surg. Pathol. 2001, 9, 201–206.
- Collins, L.C.; Achacoso, N.A.; Nekhlyudov, L.; Fletcher, S.W.; Haque, R.; Quesenberry, C.P.; Alshak, N.S.; Puligandla, B.; Brodsky, G.L.; Schnitt, S.J.; et al. Clinical and Pathologic Features of Ductal Carcinoma in Situ Associated with the Presence of Flat Epithelial Atypia: An Analysis of 543 Patients. Mod. Pathol. 2007, 20, 1149–1155.
- Foote, F.W.; Stewart, F.W. Comparative Studies of Cancerous Versus Noncancerous Breasts. Ann. Surg. 1945, 121, 197–222.
- Wellings, S.R.; Jensen, H.M.; Marcum, R.G. An Atlas of Subgross Pathology of the Human Breast with Special Reference to Possible Precancerous Lesions. J. Natl. Cancer Inst. 1975, 55, 231–273.
- Lee, S.; Mohsin, S.K.; Mao, S.; Hilsenbeck, S.G.; Medina, D.; Allred, D.C. Hormones, Receptors, and Growth in Hyperplastic Enlarged Lobular Units: Early Potential Precursors of Breast Cancer. Breast Cancer Res. 2006, 8, R6.
- Allred, D.C.; Mohsin, S.K.; Fuqua, S.A. Histological and Biological Evolution of Human Premalignant Breast Disease. Endocr. Relat. Cancer 2001, 8, 47–61.
- Azzopardi, J.G.; Ahmed, A.; Millis, R.R. Problems in Breast Pathology. Major Probl. Pathol. 1979, 11, 1–466.
- Tavassoli, F.A. Ductal Intraepithelial Neoplasia of the Breast. Virchows Arch. 2001, 438, 221–227.
- Tavassoli, F.A.; Devilee, P. Pathology and Genetics of Tumours of the Breast and Female Genitale Organs; World Health Organization Classification of Tumours: Geneva, Switzerland; International Agency for Research on Cancer: Lyon, France, 2003; ISBN 978-92-832-2412-9.
- Schnitt, S.J.; Vincent-Salomon, A. Columnar Cell Lesions of the Breast. Adv. Anat. Pathol. 2003, 10, 113–124.
- Simpson, P.T.; Gale, T.; Reis-Filho, J.S.; Jones, C.; Parry, S.; Sloane, J.P.; Hanby, A.; Pinder, S.E.; Lee, A.H.S.; Humphreys, S.; et al. Columnar Cell Lesions of the Breast: The Missing Link in Breast Cancer Progression? A Morphological and Molecular Analysis. Am. J. Surg. Pathol. 2005, 29, 734–746.
- Goldstein, N.S.; Kestin, L.J.; Vicini, F.A. Monomorphic Epithelial Proliferations: Characterization and Evidence Suggesting They Are the Pool of Partially Transformed Lesions from Which Some Invasive Carcinomas Arise. Am. J. Clin. Pathol. 2007, 128, 1023–1034.
- Shaaban, A.M.; Sloane, J.P.; West, C.R.; Moore, F.R.; Jarvis, C.; Williams, E.M.I.; Foster, C.S. Histopathologic Types of Benign Breast Lesions and the Risk of Breast Cancer: Case-Control Study. Am. J. Surg. Pathol. 2002, 26, 421–430.
- Kunju, L.P.; Kleer, C.G. Significance of Flat Epithelial Atypia on Mammotome Core Needle Biopsy: Should It Be Excised? Hum. Pathol. 2007, 38, 35–41.
- Rosen, P.P.; Hoda, S.A.; Brogi, E.; Koerner, F.C. Rosen’s Breast Pathology, 4th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2014; ISBN 978-1-4511-7653-7.
- Koerner, F.C. Diagnostic Problems in Breast Pathology; Saunders/Elsevier: Philadelphia, PA, USA, 2009; ISBN 978-1-4160-2612-9.
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. (Eds.) World Health Organization Classification of Tumours of the Breast, 4th ed.; IARC Press: Lyon, France, 2012.
- De Boer, M.; Verschuur-Maes, A.H.J.; Buerger, H.; Moelans, C.B.; Steenkamer, M.; Savola, S.; van Diest, P.J. Role of Columnar Cell Lesions in Breast Carcinogenesis: Analysis of Chromosome 16 Copy Number Changes by Multiplex Ligation-Dependent Probe Amplification. Mod. Pathol. 2018, 31, 1816–1833.
- De Boer, M.; van Diest, P.J. Blunt Duct Adenosis: A Separate Entity from Columnar Cell Lesions? J. Clin. Pathol. 2022, 75, 5–9.
- Grabenstetter, A.; Brennan, S.; Salagean, E.D.; Morrow, M.; Brogi, E. Flat Epithelial Atypia in Breast Core Needle Biopsies with Radiologic-Pathologic Concordance: Is Excision Necessary? Am. J. Surg. Pathol. 2020, 44, 182–190.
- Solorzano, S.; Mesurolle, B.; Omeroglu, A.; El Khoury, M.; Kao, E.; Aldis, A.; Meterissian, S. Flat Epithelial Atypia of the Breast: Pathological-Radiological Correlation. AJR Am. J. Roentgenol. 2011, 197, 740–746.
- Senetta, R.; Campanino, P.P.; Mariscotti, G.; Garberoglio, S.; Daniele, L.; Pennecchi, F.; Macrì, L.; Bosco, M.; Gandini, G.; Sapino, A. Columnar Cell Lesions Associated with Breast Calcifications on Vacuum-Assisted Core Biopsies: Clinical, Radiographic, and Histological Correlations. Mod. Pathol. 2009, 22, 762–769.
- Martel, M.; Barron-Rodriguez, P.; Tolgay Ocal, I.; Dotto, J.; Tavassoli, F.A. Flat DIN 1 (Flat Epithelial Atypia) on Core Needle Biopsy: 63 Cases Identified Retrospectively among 1751 Core Biopsies Performed over an 8-Year Period (1992–1999). Virchows Arch. 2007, 451, 883–891.
- Becker, A.K.; Gordon, P.B.; Harrison, D.A.; Hassell, P.R.; Hayes, M.M.; van Niekerk, D.; Wilson, C.M. Flat Ductal Intraepithelial Neoplasia 1A Diagnosed at Stereotactic Core Needle Biopsy: Is Excisional Biopsy Indicated? AJR Am. J. Roentgenol. 2013, 200, 682–688.
- Lavoué, V.; Roger, C.M.; Poilblanc, M.; Proust, N.; Monghal-Verge, C.; Sagan, C.; Tas, P.; Mesbah, H.; Porée, P.; Gay, C.; et al. Pure Flat Epithelial Atypia (DIN 1a) on Core Needle Biopsy: Study of 60 Biopsies with Follow-up Surgical Excision. Breast Cancer Res. Treat. 2011, 125, 121–126.
- Rakha, E.A.; Lee, A.H.S.; Jenkins, J.A.; Murphy, A.E.; Hamilton, L.J.; Ellis, I.O. Characterization and Outcome of Breast Needle Core Biopsy Diagnoses of Lesions of Uncertain Malignant Potential (B3) in Abnormalities Detected by Mammographic Screening. Int. J. Cancer 2011, 129, 1417–1424.
- Elif, A.; Burcu, S.; Nazan, C.; Sumru, C.Z.; Kemal, A.N. Columnar Cell Lesions of the Breast: Radiological Features and Histological Correlation. Med. Ultrason. 2015, 17, 147–154.
- Prowler, V.L.; Joh, J.E.; Acs, G.; Kiluk, J.V.; Laronga, C.; Khakpour, N.; Lee, M.C. Surgical Excision of Pure Flat Epithelial Atypia Identified on Core Needle Breast Biopsy. Breast 2014, 23, 352–356.
- Santucci, D.; Faiella, E.; Calabrese, A.; Favale, L.; Zobel, B.B.; de Felice, C. Our Radiological Experience on B3 Lesions: Correlation Between Mammographic and MRI Findings with Histologic Definitive Result. Clin. Breast Cancer 2019, 19, e643–e653.
- Saladin, C.; Haueisen, H.; Kampmann, G.; Oehlschlegel, C.; Seifert, B.; Rageth, L.; Rageth, C.; Stadlmann, S.; Kubik-Huch, R.A.; On behalf of the MIBB Group. Lesions with Unclear Malignant Potential (B3) after Minimally Invasive Breast Biopsy: Evaluation of Vacuum Biopsies Performed in Switzerland and Recommended Further Management. Acta Radiol. 2016, 57, 815–821.
- Peres, A.; Barranger, E.; Becette, V.; Boudinet, A.; Guinebretiere, J.-M.; Cherel, P. Rates of Upgrade to Malignancy for 271 Cases of Flat Epithelial Atypia (FEA) Diagnosed by Breast Core Biopsy. Breast Cancer Res. Treat. 2012, 133, 659–666.
- Srour, M.K.; Donovan, C.; Chung, A.; Harit, A.; Dadmanesh, F.; Giuliano, A.E.; Amersi, F. Flat Epithelial Atypia on Core Needle Biopsy Does Not Always Mandate Excisional Biopsy. Breast J. 2020, 26, 679–684.
- Dialani, V.; Venkataraman, S.; Frieling, G.; Schnitt, S.J.; Mehta, T.S. Does Isolated Flat Epithelial Atypia on Vacuum-Assisted Breast Core Biopsy Require Surgical Excision? Breast J. 2014, 20, 606–614.
- Okamoto, S.; Chen, S.-T.; Covelli, J.D.; DeMartini, W.B.; Daniel, B.L.; Ikeda, D.M. High-Risk Lesions Diagnosed at MRI-Guided Vacuum-Assisted Breast Biopsy: Imaging Characteristics, Outcome of Surgical Excision or Imaging Follow-Up. Breast Cancer 2020, 27, 405–414.
- Tozaki, M.; Yamashiro, N.; Sakamoto, M.; Sakamoto, N.; Mizuuchi, N.; Fukuma, E. Magnetic Resonance-Guided Vacuum-Assisted Breast Biopsy: Results in 100 Japanese Women. Jpn. J. Radiol. 2010, 28, 527–533.
- Crystal, P.; Sadaf, A.; Bukhanov, K.; McCready, D.; O’Malley, F.; Helbich, T.H. High-Risk Lesions Diagnosed at MRI-Guided Vacuum-Assisted Breast Biopsy: Can Underestimation Be Predicted? Eur. Radiol. 2011, 21, 582–589.
- Speer, M.E.; Huang, M.L.; Dogan, B.E.; Adrada, B.E.; Candelaria, R.P.; Hess, K.R.; Hansakul, P.; Yang, W.T.; Rauch, G.M. High Risk Breast Lesions Identified on MRI-Guided Vacuum-Assisted Needle Biopsy: Outcome of Surgical Excision and Imaging Follow-Up. Br. J. Radiol. 2018, 91, 20180300.
- Michaels, A.Y.; Ginter, P.S.; Dodelzon, K.; Naunheim, M.R.; Abbey, G.N. High-Risk Lesions Detected by MRI-Guided Core Biopsy: Upgrade Rates at Surgical Excision and Implications for Management. AJR Am. J. Roentgenol. 2021, 216, 622–632.
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic Density and Breast Cancer Risk: Current Understanding and Future Prospects. Breast Cancer Res. 2011, 13, 223.
- Turashvili, G.; McKinney, S.; Martin, L.; Gelmon, K.A.; Watson, P.; Boyd, N.; Aparicio, S. Columnar Cell Lesions, Mammographic Density and Breast Cancer Risk. Breast Cancer Res. Treat. 2009, 115, 561–571.
- Ghosh, K.; Vierkant, R.A.; Frank, R.D.; Winham, S.; Visscher, D.W.; Pankratz, V.S.; Scott, C.G.; Brandt, K.; Sherman, M.E.; Radisky, D.C.; et al. Association between Mammographic Breast Density and Histologic Features of Benign Breast Disease. Breast Cancer Res. 2017, 19, 134.
- Katayama, A.; Toss, M.S.; Parkin, M.; Sano, T.; Oyama, T.; Quinn, C.M.; Ellis, I.O.; Rakha, E.A. Nuclear Morphology in Breast Lesions: Refining Its Assessment to Improve Diagnostic Concordance. Histopathology 2022, 80, 515–528.
- Yamashita, Y.; Ichihara, S.; Moritani, S.; Yoon, H.-S.; Yamaguchi, M. Does Flat Epithelial Atypia Have Rounder Nuclei than Columnar Cell Change/Hyperplasia? A Morphometric Approach to Columnar Cell Lesions of the Breast. Virchows Arch. 2016, 468, 663–673.
- Lim, C.N.; Ho, B.C.S.; Bay, B.H.; Yip, G.; Tan, P.H. Nuclear Morphometry in Columnar Cell Lesions of the Breast: Is It Useful? J. Clin. Pathol. 2006, 59, 1283–1286.
- Samples, L.S.; Rendi, M.H.; Frederick, P.D.; Allison, K.H.; Nelson, H.D.; Morgan, T.R.; Weaver, D.L.; Elmore, J.G. Surgical Implications and Variability in the Use of the Flat Epithelial Atypia Diagnosis on Breast Biopsy Specimens. Breast 2017, 34, 34–43.
- Tan, P.H.; Ho, B.C.-S.; Selvarajan, S.; Yap, W.M.; Hanby, A. Pathological Diagnosis of Columnar Cell Lesions of the Breast: Are There Issues of Reproducibility? J. Clin. Pathol. 2005, 58, 705–709.
- Gomes, D.S.; Porto, S.S.; Balabram, D.; Gobbi, H. Inter-Observer Variability between General Pathologists and a Specialist in Breast Pathology in the Diagnosis of Lobular Neoplasia, Columnar Cell Lesions, Atypical Ductal Hyperplasia and Ductal Carcinoma in Situ of the Breast. Diagn. Pathol. 2014, 9, 121.
- O’Malley, F.P.; Mohsin, S.K.; Badve, S.; Bose, S.; Collins, L.C.; Ennis, M.; Kleer, C.G.; Pinder, S.E.; Schnitt, S.J. Interobserver Reproducibility in the Diagnosis of Flat Epithelial Atypia of the Breast. Mod. Pathol. 2006, 19, 172–179.
- Darvishian, F.; Singh, B.; Simsir, A.; Ye, W.; Cangiarella, J.F. Atypia on Breast Core Needle Biopsies: Reproducibility and Significance. Ann. Clin. Lab. Sci. 2009, 39, 270–276.
- Haupt, B.; Schwartz, M.R.; Xu, Q.; Ro, J.Y. Columnar Cell Lesions: A Consensus Study among Pathology Trainees. Hum. Pathol. 2010, 41, 895–901.
- Saqi, A.; Mazziotta, R.; Hamele-Bena, D. Columnar Cell Lesions: Fine-Needle Aspiration Biopsy Features. Diagn. Cytopathol. 2004, 31, 370–375.
- Jensen, K.C.; Kong, C.S. Cytologic Diagnosis of Columnar-Cell Lesions of the Breast. Diagn. Cytopathol. 2007, 35, 73–79.
- Tremblay, G.; Deschênes, J.; Alpert, L.; Quenneville, L.A.M. Overexpression of Estrogen Receptors in Columnar Cell Change and in Unfolding Breast Lobules. Breast J. 2005, 11, 326–332.
- Dessauvagie, B.F.; Zhao, W.; Heel-Miller, K.A.; Harvey, J.; Bentel, J.M. Characterization of Columnar Cell Lesions of the Breast: Immunophenotypic Analysis of Columnar Alteration of Lobules with Prominent Apical Snouts and Secretions. Hum. Pathol. 2007, 38, 284–292.
- Vincent-Salomon, A. Columnar lesions: A frequent diagnosis in breast pathology! Ann. Pathol. 2003, 23, 593–596.
- Otterbach, F.; Bànkfalvi, A.; Bergner, S.; Decker, T.; Krech, R.; Boecker, W. Cytokeratin 5/6 Immunohistochemistry Assists the Differential Diagnosis of Atypical Proliferations of the Breast. Histopathology 2000, 37, 232–240.
- Kusama, R.; Fujimori, M.; Matsuyama, I.; Fu, L.; Ishii, K.; Hama, Y.; Asanuma, K.; Shingu, K.; Kobayashi, S.; Tsuchiya, S. Clinicopathological Characteristics of Atypical Cystic Duct (ACD) of the Breast: Assessment of ACD as a Precancerous Lesion. Pathol. Int. 2000, 50, 793–800.
- De Potter, C.R.; Foschini, M.P.; Schelfhout, A.M.; Schroeter, C.A.; Eusebi, V. Immunohistochemical Study of Neu Protein Overexpression in Clinging in Situ Duct Carcinoma of the Breast. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 422, 375–380.
- Noel, J.-C.; Fayt, I.; Fernandez-Aguilar, S.; Buxant, F.; Boutemy, R. Proliferating Activity in Columnar Cell Lesions of the Breast. Virchows Arch. 2006, 449, 617–621.
- Tomasino, R.M.; Morello, V.; Gullo, A.; Pompei, G.; Agnese, V.; Russo, A.; Rinaldi, G. Assessment of “Grading” with Ki-67 and c-Kit Immunohistochemical Expressions May Be a Helpful Tool in Management of Patients with Flat Epithelial Atypia (FEA) and Columnar Cell Lesions (CCLs) on Core Breast Biopsy. J. Cell. Physiol. 2009, 221, 343–349.
This entry is offline, you can click here to edit this entry!
