Among numerous synthetic macromolecules, polyurethane in its different forms has proven its sheer dominance and established a reputation as a reliable and trusted material due to its proficiency in terms of superior properties, which include: high mechanical strength and abrasion resistance, good durability, good adhesion, good thermal stability, excellent chemical and weathering resistance. Synthetic polyurethane materials are non-biodegradable, poisonous, and use petrochemical-based raw materials, which are now depleting, leading to a surge in polyurethane production costs. Enormous kinds of available bio-renewable sources as predecessors for the production of polyols and isocyanates have been explored for the development of “greener” PU materials; these bio-based polyurethanes have significant potential to be used as future PU products, with a partial or total replacement of petroleum-based polyurethanes, due to increasing concern about the environment, their relatively low cost and biodegradability.
- polyurethanes
- non-isocyanate polyurethanes
- adhesives
- coatings
- recycling
- biodegradation
1. Introduction
| Thermoplastic PU | Flexible PU | Rigid PU | Polyurethane Ionomers |
Water-Based PU |
|---|---|---|---|---|
 |
 |
 |
 |
 |
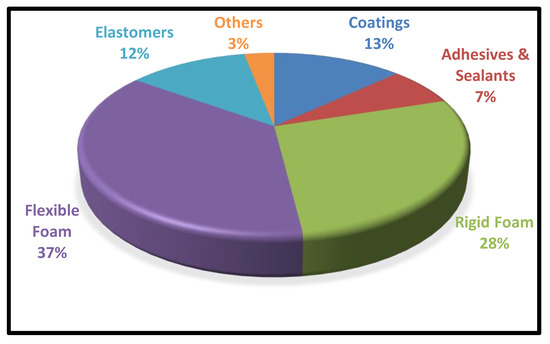
| Flexible and elastic with good abrasion, weather, and abrasion resistance. | Block copolymers with phase separation between soft and hard segments that are flexible. | Versatile and energy-saving insulator | Improved thermal and mechanical characteristics and greater dispersion in polar liquids due to increased hydrophilicity. | Coatings and adhesives which use water as solvent are called water-based polyurethane. |
| For example, automotive instruments, footwear, medical devices, film, sheet, and applications with a profile. | For example, cushion materials, carpet underlays, furniture, packaging, biomedical, and industries that use cushion materials. | For example, sound and thermal insulators. Used in commercial and residential appliances. | For example, biomedical devices, shape memory and are biocompatible. | For example, coatings, adhesives, sealants, binders |
1.1. Bio-Based Polyurethane
| Property | Bio-Based Polyurethanes | Petro-Based Polyurethanes |
|---|---|---|
| Density (kg m−3) | 112–181 | 141–181 |
| Thermal Conductivity (Wm−1K−1) | 0.06–0.0540 | 0.0540 |
| Volatile Organic Compound (µg m−3) | Not detected at 61–91 °C | Not detected at 61–91 °C |
| Compressive Strength (N mm−2) | 111–171 | 58.0 |
| Tensile Strength (N mm−2) | 1.4–1.6 | 0.84 |
| Bending strength (N mm−2) | 2.7–3.5 | 1.88 |
| Bending Stress (N mm−2) | 1.55 | 1.50 |
| Water Absorption (kg m−3) | 0.3 | 0.24 |
1.1.1. Vegetable Oil-Derived Monomers
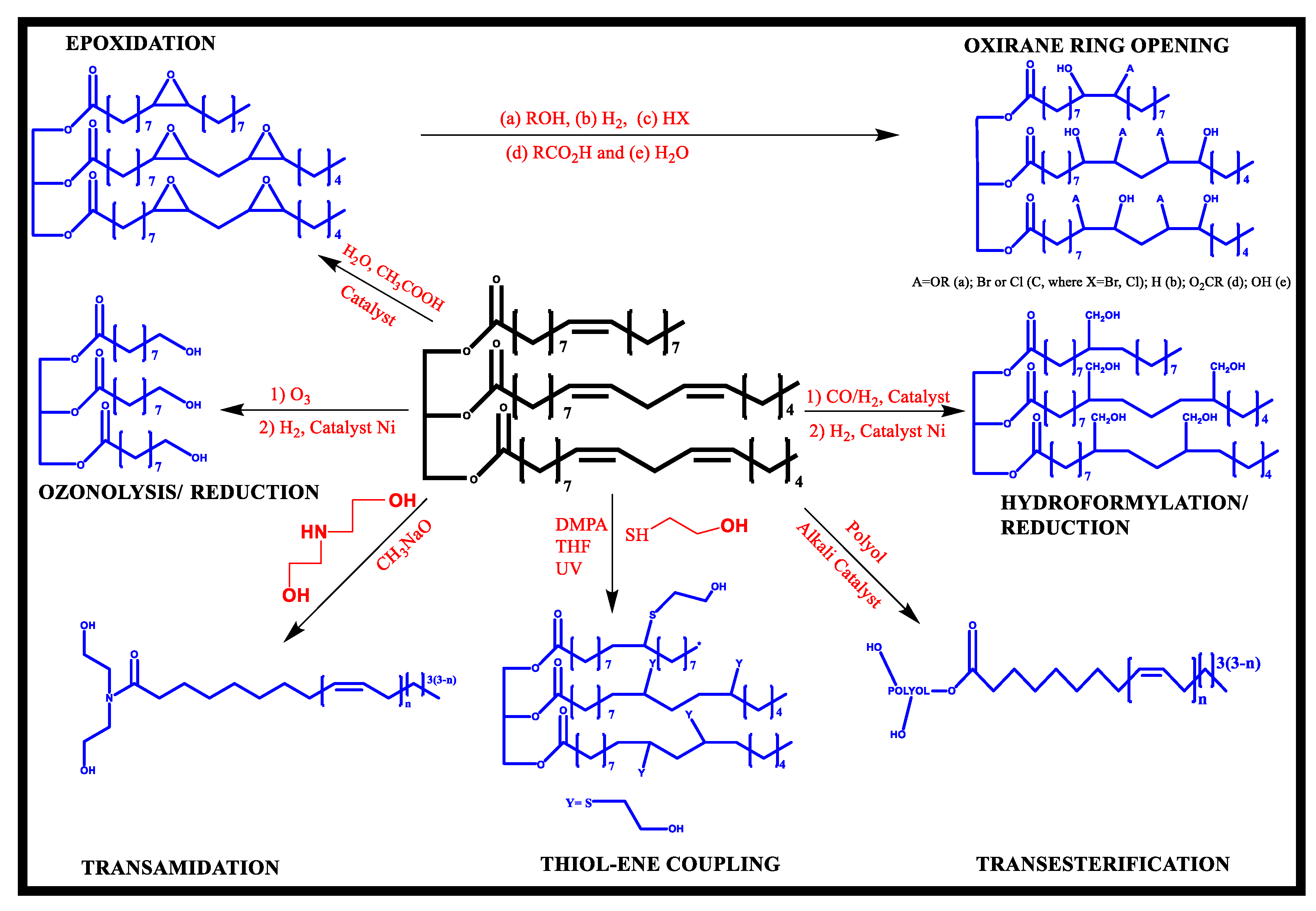
1.1.2. Bio-Based Isocyanates
2. Polyurethanes: Types, Synthesis, and Utilities
2.1. Polyurethane Adhesives
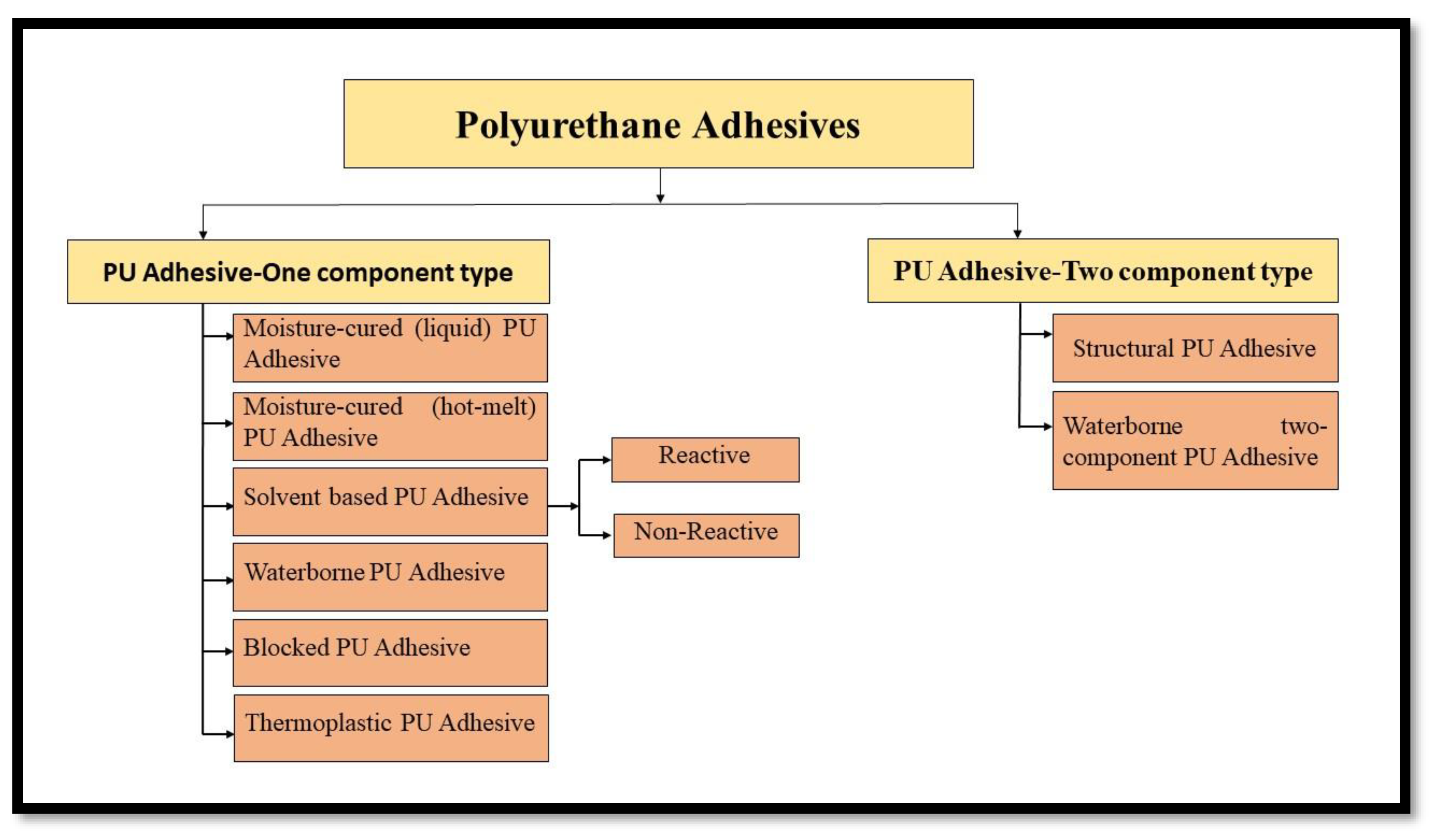
2.1.1. Protein
| Animal-Based Adhesives |
Advantages | Disadvantages |
|---|---|---|
| Tissue and bones | Cheap, non-toxic, sets quickly, has a high shear strength, and doesn’t discolour wood | Has low water and damp resistance and does not produce distinct adhesive lines |
| Albumin and blood | Rapid heat setting, excellent dry shear strength, microorganism resistance, and does not discolour wood | Suitable to glue thin sheets |
| Casein | Resistance to water, damp conditions, elevated temperature, high shear strength | May discolour woods, dissolves only at high pH, expensive, not suitable for exterior use |
| Plant-Based Adhesives |
Advantages | Disadvantages |
| Soybean | Non-toxic, has strong dry strength, and is moderately resistant to heat, wet, and damp environments. | Denaturation of proteins in alkaline solution which is caustic and discolour wood, sensitive to microbial degradation, and not suitable for exterior applications |
| Peanut | Non-toxic, hygroscopic, superiority of colour | Low shear strength, produces blisters, small bubble-like voids, and peanut allergies limit its applications. |
| Gluten | Non-toxic, sustainable source of protein | Costly |
2.1.2. Vegetable Oils
Castor Oil
| S. No. | Compositions of PU Adhesive | Method of Preparation | Applications |
|---|---|---|---|
| 1. | Castor oil—pentaerythritol | Trans esterification reaction | Bonding/adhesion of wood to wood and metal to metal |
| 2. | Castor oil—polyester polyols | Trans esterification reaction | Wood adhesive |
| 3. | Castor oil—cellulose acetate | Condensation reaction | Wood-wood, steel-steel adhesion |
| 4. | Castor oil—MDI modified cellulose acetate | Condensation reaction | Wood-wood, stainless steel lubricants |
| 5. | Castor oil-based PU adhesives | Condensation/trans esterification reactions | Wood adhesives, sealants |
| 6. | Castor oil—dicarboxylic acid (maleic acid, fumaric acid, oxalic acid) | Condensation reaction | Wood Adhesives |
Palm Oil
Canola Oil
Jatropha Oil
Soybean Oil
2.1.3. Lignin
2.1.4. Starch
2.1.5. Polylactic Acid
2.1.6. Applications of Bio-Based PU Adhesive
2.2. Polyurethane Coatings
2.2.1. Vegetable Oil-Based Polyurethane Coatings
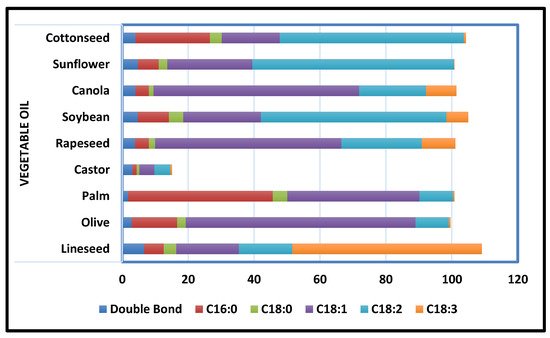
2.2.2. PU Coatings Based on Cashew Nut Shell Liquid (CNSL)
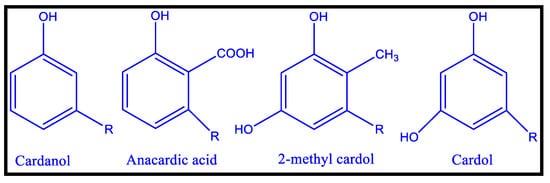
2.2.3. Polyurethane Coatings Based on Terpene
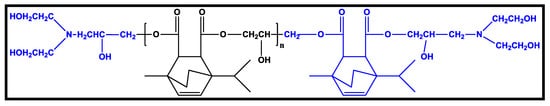
2.2.4. Polyurethane Coatings Based on Eucalyptus Tar
This entry is adapted from the peer-reviewed paper 10.3390/macromol2030019
References
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2020, 278, 111527.
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482.
- Cornille, A.; Guillet, C.; Benyahya, S.; Negrell, C.; Boutevin, B.; Caillol, S. Room temperature flexible isocyanate-free polyurethane foams. Eur. Polym. J. 2016, 84, 873–888.
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526.
- Kulkarni, R.D.; Paraskar, P.M. Synthesis of Isostearic Acid/Dimer Fatty Acid-Based Polyesteramide Polyol for the Development of Green Polyurethane Coatings. J. Polym. Environ. 2021, 29, 54–70.
- Cheng, Z.; Li, Q.; Yan, Z.; Liao, G.; Zhang, B.; Yu, Y.; Yi, C.; Xu, Z. Design and synthesis of novel aminosiloxane crosslinked linseed oil-based waterborne polyurethane composites and its physicochemical properties. Prog. Org. Coat. 2018, 127, 194–201.
- Kovács, E.; Turczel, G.; Szabó, L.; Varga, R.; Tóth, I.; Anastas, P.T.; Tuba, R. Synthesis of 1,6-Hexandiol, Polyurethane Monomer Derivatives via Isomerization Metathesis of Methyl Linolenate. ACS Sustain. Chem. Eng. 2017, 5, 11215–11220.
- Liang, H.; Feng, Y.; Lu, J.; Liu, L.; Yang, Z.; Luo, Y.; Zhang, Y.; Zhang, C. Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils. Ind. Crops Prod. 2018, 122, 448–455.
- Gomez-Jimenez-Aberasturi, O.; Gomez, J.R. New approaches to producing polyols from biomass. J. Chem. Technol. Biotechnol. 2017, 92, 705–711.
- Wang, Y.; Deng, L.; Fan, Y. Preparation of Soy-Based Adhesive Enhanced by Waterborne Polyurethane: Optimization by Response Surface Methodology. Adv. Mater. Sci. Eng. 2018, 2018, 9253670.
- Zuber, M.; Shah, S.A.A.; Jamil, T.; Asghar, M.I. Performance behavior of modified cellulosic fabrics using polyurethane acrylate copolymer. Int. J. Biol. Macromol. 2014, 67, 254–259.
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crop. Prod. 2015, 76, 215–229.
- Vanbésien, T.; Monflier, E.; Hapiot, F. Hydroformylation of vegetable oils: More than 50 years of technical innovation, successful research, and development. Eur. J. Lipid Sci. Technol. 2015, 118, 26–35.
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267.
- Peyrton, J.; Chambaretaud, C.; Sarbu, A.; Avérous, L. Biobased Polyurethane Foams Based on New Polyol Architectures from Microalgae Oil. ACS Sustain. Chem. Eng. 2020, 8, 12187–12196.
- Devi, K.; Nurul, P.P.; Ain, H.; Tuan Noor Maznee, T.I.; MohdNorhisham, S.; Srihanum, A.; Norhayati, M.N.; Yeong, S.K.; Hazimah, A. Green Polyurethane for Ornamental Products; MPOB Information Series, MPOB TT No. 522; MPOB: Bandar Baru Bangi, Malaysia, 2012.
- De Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. ACS Symp. Ser. 2021, 1380, 1–24.
- KhoonPoh, A.; Choy Sin, L.; Foon, C.; Hock, C. Polyurethane wood adhesive from palm oil-based polyester polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033.
- Ang, K.P.; Lee, C.S.; Cheng, S.F.; Chuah, C.H. Synthesis of palm oil-based polyester polyol for polyurethane adhesive production. J. Appl. Polym. Sci. 2013, 131, 6.
- Li, N.; Qi, G.; Sun, X.S.; Stamm, M.J.; Wang, D. Physicochemical Properties and Adhesion Performance of Canola Protein Modified with Sodium Bisulfite. J. Am. Oil Chem. Soc. 2011, 89, 897–908.
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185.
- Saalah, S.; Abdullah, L.; Aung, M.; Salleh, M.; Biak, D.A.; Basri, M.; Jusoh, E.; Mamat, S.; Al Edrus, S.O. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795.
- Wang, C.-S.; Yang, L.-T.; Ni, B.-L.; Shi, G. Polyurethane networks from different soy-based polyols by the ring opening of epoxidized soybean oil with methanol, glycol, and 1,2-propanediol. J. Appl. Polym. Sci. 2009, 114, 125–131.
- Valero, M.; Gonzalez, A. Polyurethane adhesive system from castor oil modified by a transesterification reaction. J. Elastomers Plast. 2012, 44, 433–442.
- Wang, Z.; Yu, L.; Ding, M.; Tan, H.; Li, J.; Fu, Q. Preparation and rapid degradation of nontoxic biodegradable polyurethanes based on poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) and l-lysine diisocyanate. Polym. Cem. 2011, 2, 601–607.
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Evaluation of biodegradability of green polyurethane/nanosilica composite synthesized from transesterified castor oil and palm oil based isocyanate. Int. Biodeterior. Biodegrad. 2017, 117, 278–288.
- Rodriguez, R.; Pérez, B.; Florez, S. Effect of Different Nanoparticles on Mechanical Properties and Curing Behavior of Thermoset Polyurethane Adhesives. J. Adhes. 2014, 90, 848–859.
- Mirabedini, S.; Mohseni, M.; PazokiFard, S.; Esfandeh, M. Effect of TiO2 on the mechanical and adhesion properties of RTV silicone elastomer coatings. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 80–86.
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202.
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685.
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108.
- Liu, X.; Hong, W.; Chen, X. Continuous Production of Water-Borne Polyurethanes: A Review. Polymers 2020, 12, 2875.
- Fuensanta, M.; Martin-Martínez, J.M. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2019, 88, 81–90.
- Mehdizadeh, M.; Yang, J. Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 2013, 13, 271–288.
- Zhou, X.; Tu, W.; Hu, J. Preparation and Characterization of Two-component Waterborne Polyurethane Comprised of Water-soluble Acrylic Resin and HDI Biuret. Chin. J. Chem. Eng. 2006, 14, 99–104.
- Malik, M.; Kaur, R. Mechanical and Thermal Properties of Castor Oil–Based Polyurethane Adhesive: Effect of TiO2 Filler. Adv. Polym. Technol. 2016, 37, 24–30.
- Dai, J.; Liu, X.; Ma, S.; Wang, J.; Shen, X.; You, S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215.
- Raydan, N.D.V.; Leroyer, L.; Charrier, B.; Robles, E. Recent Advances on the Development of Protein-Based Adhesives for Wood Composite Materials—A Review. Molecules 2021, 26, 7617.
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Processes 2016, 4, 11.
- Silva, B.B.R.; Santana, R.M.C.; Forte, M.C. A solventless castor oil-based PU adhesive for wood and foam substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565.
- Patel, M.R.; Shukla, J.M.; Patel, N.K.; Patel, K.H. Biomaterial based novel polyurethane adhesives for wood to wood and metal to metal bonding. Mater. Res. 2009, 12, 385–393.
- Radojčić, D.; Ionescu, M.; Petrović, Z.S. Novel potentially biodegradable polyurethanes from bio-based polyols. Contemp. Mater. 2013, 4, 9–21.
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Bio-Renewable Sources for Synthesis of Eco-Friendly Polyurethane Adhesives—Review. Open J. Polym. Chem. 2017, 7, 57–75.
- Börcsök, Z.; Pásztory, Z. The role of lignin in wood working processes using elevated temperatures: An abbreviated literature survey. Eur. J. Wood Wood Prod. 2021, 79, 511–526.
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18.
- Sudoł, E.; Kozikowska, E. Mechanical Properties of Polyurethane Adhesive Bonds in a Mineral Wool-Based External Thermal Insulation Composite System for Timber Frame Buildings. Materials 2021, 14, 2527.
- Calpena, E.O.; Francisca, A.A.; Sánchez, M.A.M. Adhesives in the Footwear Industry: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 69–91.
- Bao, Z.; Gao, M.; Sun, Y.; Nian, R.; Xian, M. The recent progress of tissue adhesives in design strategies, adhesive mechanism and applications. Mater. Sci. Eng. C 2020, 111, 110796.
- Liu, L.; Lu, J.; Zhang, Y.; Liang, H.; Liang, D.; Jiang, J.; Lu, Q.; Quirino, R.L.; Zhang, C. Thermosetting polyurethanes prepared with the aid of a fully bio-based emulsifier with high bio-content, high solid content, and superior mechanical properties. Green Chem. 2018, 21, 526–537.
- Chattopadhyay, D.; Kothapalli, R.V. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418.
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184.
- Hu, Y.; Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Shang, Q.; An, R.; Liu, C.; Hu, L.; Zhou, Y. Rubber Seed Oil-Based UV-Curable Polyurethane Acrylate Resins for Digital Light Processing (DLP) 3D Printing. Molecules 2021, 26, 5455.
- Patil, A.M.; Jirimali, H.D.; Gite, V.V.; Jagtap, R.N. Synthesis and performance of bio-based hyperbranched polyol in polyurethane coatings. Prog. Org. Coat. 2020, 149, 105895.
- Jeon, I.-Y.; Noh, H.-J.; Baek, J.-B. Hyperbranched Macromolecules: From Synthesis to Applications. Molecules 2018, 23, 657.
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent trends in environmentally friendly water-borne polyurethane coatings: A review. Korean J. Chem. Eng. 2015, 33, 388–400.
- Anıl, D.; Berksun, E.; Durmuş-Sayar, A.; Sevinis-Özbulut, E.B.; Ünal, S. Chapter 11—Recent advances in waterborne polyurethanes and their nanoparticle-containing dispersions. In Handbook of Waterborne Coatings; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–302.
- Zhang, Y.; Zhang, W.; Wang, X.; Dong, Q.; Zeng, X.; Quirino, R.L.; Lu, Q.; Wang, Q.; Zhang, C. Waterborne polyurethanes from castor oil-based polyols for next generation of environmentally-friendly hair-styling agents. Prog. Org. Coat. 2020, 142, 105588.
- Agnol, L.D.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-curable waterborne polyurethane coatings: A state-of-the-art and recent advances review. Prog. Org. Coat. 2021, 154, 106156.
- Ghosh, T.; Karak, N. Cashew nut shell liquid terminated self-healable polyurethane as an effective anticorrosive coating with biodegradable attribute. Prog. Org. Coat. 2019, 139, 105472.
- Kathalewar, M.; Sabnis, A.; D’Mello, D. Isocyanate free polyurethanes from new CNSL based bis-cyclic carbonate and its application in coatings. Eur. Polym. J. 2014, 57, 99–108.
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153.
- Kinaci, E.; Can, E.; La Scala, J.J.; Palmese, G.R. Epoxidation of Cardanol’s Terminal Double Bond. Polymers 2020, 12, 2104.
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials 2022, 15, 2308.
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497.
- Liu, G.; Wu, G.; Jin, C.; Kong, Z. Preparation and antimicrobial activity of terpene-based polyurethane coatings with carbamate group-containing quaternary ammonium salts. Prog. Org. Coat. 2015, 80, 150–155.
- Llevot, A.; Meier, M. Perspective: Green polyurethane synthesis for coating applications. Polym. Int. 2019, 68, 826–831.
- Olcay, H.; Kocak, E.D.; Yıldız, Z. Sustainability in Polyurethane Synthesis and Bio-based Polyurethanes BT. In Sustainability in the Textile and Apparel Industries: Sourcing Synthetic and Novel Alternative Raw Materials; Springer International Publishing: Cham, Switzerland, 2020; pp. 139–156.
