Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Among numerous synthetic macromolecules, polyurethane in its different forms has proven its sheer dominance and established a reputation as a reliable and trusted material due to its proficiency in terms of superior properties, which include: high mechanical strength and abrasion resistance, good durability, good adhesion, good thermal stability, excellent chemical and weathering resistance. Synthetic polyurethane materials are non-biodegradable, poisonous, and use petrochemical-based raw materials, which are now depleting, leading to a surge in polyurethane production costs.
- polyurethanes
- non-isocyanate polyurethanes
- adhesives
- coatings
- recycling
- biodegradation
1. Introduction
Polyurethanes (PU) represent nearly 8% of the total plastics production and stand tall as the 6th most widely used polymer in the world, with total production reaching 18 MTon in 2016 [1] and still progressing. The superior properties of PU, such as flexibility, corrosion, resistance to chemicals, high automatic strength, low-temperature comfortability, adhesiveness, and chemical structure adaptability, etc. account for their considerable popularity among researchers. PU have urethane groups as major repeating units along their main backbone chains, which are derived by reacting hydroxyl (-OH) terminated chemical entities, with isocyanate (-NCO) terminated compounds, as represented by a generalized scheme shown in Figure 1. In addition, some other functional groups may also be present in the end constitutions of the PU chains, including ethers, esters, urea, biuret, and aromatic moieties [2].
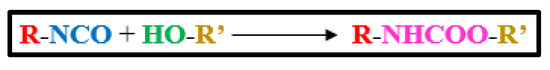
Figure 1. Formation of Urethane Linkage.
By altering the raw materials, additives, and the type of manufacturing process, the PUs can be tailor-made with a wide spectrum of product implications, including foams, fibres, adhesives, coatings, elastomers, and sealants, etc., Table 1 depicts the wide range of applications associated with polyurethanes. According to the latest report, the global polyurethane market will reach USD 88 million by 2026, with a compound annual growth rate (CGAR) of 6% [3]. The utilization of Polyurethanes in various spheres of life is represented in Figure 2.
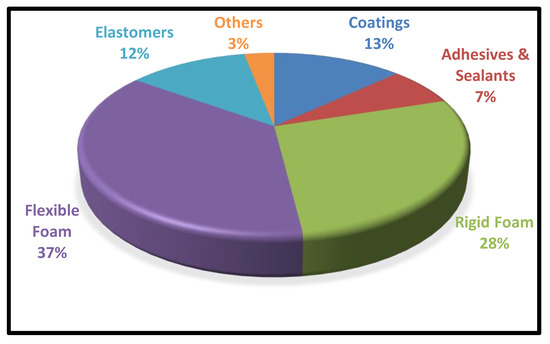
Figure 2. Global Consumption of PUs in Different Sectors.
Table 1. Wide Range of Applications Associated with Polyurethanes.
| Thermoplastic PU | Flexible PU | Rigid PU | Polyurethane Ionomers |
Water-Based PU |
|---|---|---|---|---|
 |
 |
 |
 |
 |
| Flexible and elastic with good abrasion, weather, and abrasion resistance. | Block copolymers with phase separation between soft and hard segments that are flexible. | Versatile and energy-saving insulator | Improved thermal and mechanical characteristics and greater dispersion in polar liquids due to increased hydrophilicity. | Coatings and adhesives which use water as solvent are called water-based polyurethane. |
| For example, automotive instruments, footwear, medical devices, film, sheet, and applications with a profile. | For example, cushion materials, carpet underlays, furniture, packaging, biomedical, and industries that use cushion materials. | For example, sound and thermal insulators. Used in commercial and residential appliances. | For example, biomedical devices, shape memory and are biocompatible. | For example, coatings, adhesives, sealants, binders |
The physical properties of PU, such as superb adhesion, adaptability, superior chemical resistance, and high automotive strength make it a great alternative to the widely used polymers such as formaldehyde and epoxy resins; however, the drawbacks associated with the preparation and usage of polyurethanes poses a serious threat to the human health and environmental hazards, which includes (i) the use of dangerous phosgene gas for the conversion of amines into isocyanates, (ii) usage of CMR (Carcinogenic, Mutagenic, and Reprotoxic) labelled isocyanates for PU synthesis, namely Methylene Diphenyl Diisocyanate (MDI) and Toluene Diisocyanate (TDI), and the health risks associated with their long-term exposure, such as asthma, dermatitis conjunctivitis and acute poisoning (iii) post-consumption dumping (into landfills and dumping ground) or incineration of PU, leading to either hydrolytic degradation, that generates toxic amines which contaminates air and water bodies or the disintegration to yield hydrogen cyanide (HCN), during burning [4,5].
To address these issues, a new quest has already begun and gained popularity to produce non-isocyanate-based polyurethanes (NIPU), in which the common approach for the PU synthesis involves the reaction between cyclic carbonates and amines, resulting in a class of compounds called Polyhydroxyurethane (PHU) with hydroxyl groups [6,7]. This extensive review provides a deep insight into the recent research and developments related to polyurethanes prepared from bio-based materials being used in different segments, such as adhesives, coatings, elastomers, and foams for a broad range of practical applications. In addition, the new methodologies adopted in the field of NIPU, PU recycling, and PU biodegradation have been highlighted in detail.
1.1. Bio-Based Polyurethane
Synthetic polyurethane materials are non-biodegradable, expensive, poisonous, and have petrochemical-based raw materials, which are now depleting. Bio-based polyurethanes are one of today’s most flexible materials, which can play a significant role in fostering environmental goals of promoting waste reduction and sustainability. PU has seen a significant shift from traditional petroleum-based raw feedstock toward diverse renewable alternatives such as fatty acids [8], proteins [9], carbohydrates [10], vegetableoils [11], starch [12], polysaccharides [13], cellulose [14], and a variety of different agricultural products and by-products. Using renewable resources to produce polyurethane reduces adverse influences on the environment, for instance, greenhouse gas emissions. In the recent decade, diverse research groups have made rewarding efforts toward the synthesis of biopolyol-based polyurethanes [15,16]. Polyols behave as soft segments and transmit flexibility and exhibit elastomeric properties in the polyurethanes [17]. Polyols derived from vegetable oils have been the subject of extensive research. Hydroformylation [18], ozonolysis [19], amidation-esterification [20], metathesis [21], and epoxidation-ring opening [22], coupling of thiol-ene with mercaptoethanol [23] have all been used to achieve this, and all have successfully provided polyol with the desired properties; moreover, petrochemical-based isocyanates are replaced by bio-based isocyanate to remove toxicity such as partially bio-based aliphatic isocyanate. These impart water resistance, good mechanical strength, and flexibility to the PU network [24]. Hence, bio-based polyurethane materials were created to solve challenges such as solid pollution, economic effectiveness, and easy availability of raw materials. A comparison of the properties of bio-based PU and petro-based PU is given in Table 2.
Table 2. Comparison of the Properties of Bio-Based Polyurethane and Petro-Based Polyurethane [16].
| Property | Bio-Based Polyurethanes | Petro-Based Polyurethanes |
|---|---|---|
| Density (kg m−3) | 112–181 | 141–181 |
| Thermal Conductivity (Wm−1K−1) | 0.06–0.0540 | 0.0540 |
| Volatile Organic Compound (µg m−3) | Not detected at 61–91 °C | Not detected at 61–91 °C |
| Compressive Strength (N mm−2) | 111–171 | 58.0 |
| Tensile Strength (N mm−2) | 1.4–1.6 | 0.84 |
| Bending strength (N mm−2) | 2.7–3.5 | 1.88 |
| Bending Stress (N mm−2) | 1.55 | 1.50 |
| Water Absorption (kg m−3) | 0.3 | 0.24 |
1.1.1. Vegetable Oil-Derived Monomers
Vegetable-oil-based polyols have reportedly been synthesized by ozonolysis, thiol-ene coupling process, photochemical oxidation, hydroformylation, ring-opening events, and epoxidation (Figure 3). Epoxidation is the major chemical pathway for making polyol, followed by epoxy group reactions with ring-opening reagents such as glycerol, alcohol, 1,2-propanediol, water, and acids.
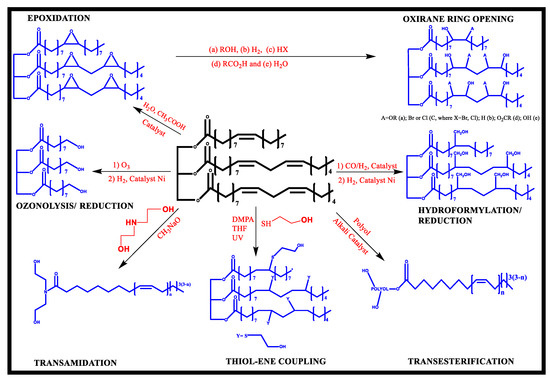
Figure 3. Synthetic pathways for the synthesis of polyols based on vegetable oil [25].
Additionally, lactate polyols, prepared from 100% bio-renewable material are polyester polyols that incorporate lactic acid units as a component. Polyurethane obtained from lactate polyols is found to be biodegradable and biocompatible. Two methods are commonly being reported for the synthesis of polyols from polylactic acids: (1) ring-opening addition (lactide is added to hydroxyl groups to open the ring). (2) Different polyols are esterified using lactic acid or Transesterification with lactic acid esters [25].
1.1.2. Bio-Based Isocyanates
To boost the renewable content of PUs, partially or potentially bio-based di-, tri- or poly-isocyanates have been utilized. There are also reports on PU films made with two bio-based isocyanates—ethyl ester L-lysine diisocyanate (LLDI) and ethyl ester l-lysine triisocyanate (LLTI)—and one commercial isocyanate, isophorone diisocyanate (IPDI) [25]. Another study documents the preparation of bio-based polyurethane by utilizing isocyanates derived from palm oil, which was reported to exhibit low toxicity, low carbon emission, along with being cost-effective [26].
2. Polyurethanes: Types, Synthesis, and Utilities
2.1. Polyurethane Adhesives
Polyurethane (PU) adhesives are known for their remarkable adhesion, weathering resistance, excellent low-temperature performance, flexibility, and rate of curing, which can be speckled according to the need of the manufacturer [27,28]. PU adhesives have advantages over other adhesives being pressure-sensitive, having high tensile strength, compressive modulus, and rip strength, and being transparent at room temperature. Owing to a vast diversity among the raw materials, the properties of the PU adhesives can be accustomed to a vast extent as per the needs of the users. The high-strength PU adhesives can be utilized in both inelastic and permanently elastic applications [29].High penetration of the adhesive into the wood, limited resistance to exfoliation, and undesired gap-filling properties are the main drawbacks of PU adhesives [30]. Most of the commercially important PU adhesives can be categorized as one component or two components of adhesives, as depicted in Figure 4.
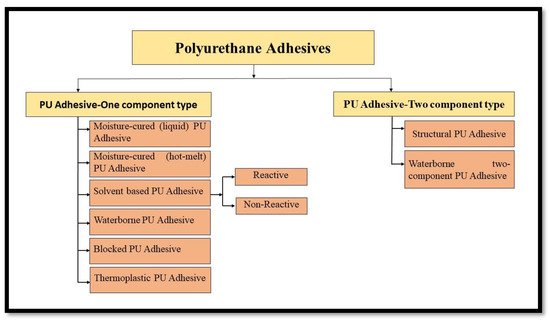
Figure 4. Classification of Polyurethane Adhesives Based on Number of Constituents.
PU Adhesives—One-component Type: One component adhesives are self-curing adhesives that do not have to be mixed with any other ingredients (curing agent) before being applied to the substrate; they use an isocyanate-terminated pre-polymer that cures naturally in the presence of moisture. After applying to substrates, a mist of water is spurted on the adhesive to speed up the curing process [30].
Other types of one-component PU adhesives include solvent-based PU adhesives, which are further categorised as reactive solvent-borne PU adhesive and non-reactive solvent-borne PU adhesive [31]. The former involves high-molecular-weight oligomers bearing isocyanate functionality and the latter shows no further reaction after application. Solvents commonly used in these adhesives are toluene, ketones, ethyl acetate, butyl acetate, etc.
Additionally, Waterborne polyurethanes are a kind of system composed of a dispersion of binary colloidal particles within an aqueous medium that necessitate the addition of water-compatible stabilising groups (emulsifiers) due to their hydrophobicity [32]. Blocked PU Adhesives, as the term ‘blocked’ implies, are the one in which one of the reactants involved is blocked chemically to inhibit the reaction. Commercially available blocked isocyanates are mostly based on TDI, and derivatives of HMDI.
The thermoplastic PU adhesives are linear in structure with a functionality equal to 2 [33]. The desired molecular weight to synthesize thermoplastic PU adhesives is about 100,000 or higher; these are superior compared to hot melt adhesives, in terms of holding strength, attained by crystallization.
PU Adhesives—Two-Component Type: Two-component PU adhesives are applied where, a better flexible bond line is desired; these are commonly used for bonding two different kinds of substrate, which exhibits a wide difference in their thermal expansion coefficients. Due to their fast reaction rate, two-component polyurethane adhesives are generally prepared with components supplied in separate compartments. The two-component PU adhesive is broadly categorized as Structural PU adhesives and Waterborne Two-Component PU adhesives.
Structural PU Adhesive provides excellent mechanical strength by forming a chemical cross-link between the reactive components, thus leading to a three-dimensional polymer network, a necessity for structural performance [34]. Waterborne Two-Component PU Adhesive consists of two components, with one of the components as PU dispersions and the other one as a crosslinker [35].
Moreover, the addition of fillers can potentially change the physicochemical and mechanical properties of adhesives. Talc, calcium carbonate, silica, titanium oxide, carbon fibre, clay, and other fillers are commonly used in PU. The effect of nanosized TiO2 filler on various characteristics of PU glue was investigated in a recent study. In PU adhesives, TiO2 has been shown to improve surface adherence, solar reflectance, and crack resistance [36].
In general, synthetic adhesives are more resistant to moisture, chemicals, and biological organisms than natural adhesives. Bio-renewable sources such as vegetable oils, starch, protein, lignin, lactic acid etc. constitute a valuable source for the synthesis of polyols to produce ‘‘sustainable, environment friendly‘’ PU adhesives [37]. Bio-based polyurethane adhesives from various sources are discussed here:
2.1.1. Protein
Protein-based PU adhesives can be prepared from both animal as well as plant sources. The advantages and disadvantages of the different animal and plant protein-based adhesives have been reported in Table 3.
Table 3. Different Animal and Plant Protein-based Adhesives [38].
| Animal-Based Adhesives |
Advantages | Disadvantages |
|---|---|---|
| Tissue and bones | Cheap, non-toxic, sets quickly, has a high shear strength, and doesn’t discolour wood | Has low water and damp resistance and does not produce distinct adhesive lines |
| Albumin and blood | Rapid heat setting, excellent dry shear strength, microorganism resistance, and does not discolour wood | Suitable to glue thin sheets |
| Casein | Resistance to water, damp conditions, elevated temperature, high shear strength | May discolour woods, dissolves only at high pH, expensive, not suitable for exterior use |
| Plant-Based Adhesives |
Advantages | Disadvantages |
| Soybean | Non-toxic, has strong dry strength, and is moderately resistant to heat, wet, and damp environments. | Denaturation of proteins in alkaline solution which is caustic and discolour wood, sensitive to microbial degradation, and not suitable for exterior applications |
| Peanut | Non-toxic, hygroscopic, superiority of colour | Low shear strength, produces blisters, small bubble-like voids, and peanut allergies limit its applications. |
| Gluten | Non-toxic, sustainable source of protein | Costly |
2.1.2. Vegetable Oils
Vegetable oils are an excellent raw material for the production of PU adhesives. The most important component of vegetable oil is triglycerides, which are glycerol esters with three long chains of fatty acids of varying composition depending on the source of the oil. As triglycerides hydrolyse to form a range of fatty acids and glycerols, polyols, and isocyanates, raw materials for the synthesis of polyurethane can be derived from them. Various vegetable oils that can be utilized for PU synthesis are Castor Oil, Palm Oil, Canola Oil, Soyabean Oil and Jatropha Oil.
Castor Oil
Of all the vegetable oils, castor oil is the favourite among the researchers and the most commonly used one because of its renewability, low cost, easy availability, and being non-edible. Castor oil contains ricinoleic acid, which is a monounsaturated fatty acid, bearing a hydroxyl functional group and thus, can be employed for PU synthesis [39]. Different compositions of PU adhesives derived from castor oil have been reported in Table 4. Being non-edible (due to the presence of toxic protein, i.e., ricin), there is no hesitation among the researchers to use it as a potential source of PU synthesis. The mechanical properties of the castor oil-based adhesive are highly dependent on the NCO/OH molar ratio, due to higher or lower cross-linking achieved during the reaction. Studies on solvent-less castor oil-based PU adhesives reported the impact of the NCO/OH molar ratio and the chemical characteristics of the substrates on the adhesion force [40]. As reported, the resulted PU adhesive foam joints displayed peeling strength values of 75% and wood joints showed lap shear strength values 20% higher than that of commercialized solvent-based adhesives.
Table 4. Different Compositions of PU Adhesives Derived from Castor Oil [35].
| S. No. | Compositions of PU Adhesive | Method of Preparation | Applications |
|---|---|---|---|
| 1. | Castor oil—pentaerythritol | Trans esterification reaction | Bonding/adhesion of wood to wood and metal to metal |
| 2. | Castor oil—polyester polyols | Trans esterification reaction | Wood adhesive |
| 3. | Castor oil—cellulose acetate | Condensation reaction | Wood-wood, steel-steel adhesion |
| 4. | Castor oil—MDI modified cellulose acetate | Condensation reaction | Wood-wood, stainless steel lubricants |
| 5. | Castor oil-based PU adhesives | Condensation/trans esterification reactions | Wood adhesives, sealants |
| 6. | Castor oil—dicarboxylic acid (maleic acid, fumaric acid, oxalic acid) | Condensation reaction | Wood Adhesives |
Palm Oil
Palm oil and palm kernel oil are the two main forms of oil generated from the palm oil tree. Palm oil has been reportedly used by researchers for the production of PU adhesives, which shows higher hydrolytic stability and hydrophobicity. Patel et al. studied the ring-opening reaction of epoxidized palm olefin with phthalic acid, which is utilized to generate PU adhesives with, which contain palm oil-based polyester [41]. Khoon et al. studied polyurethane wood adhesive derived from palm oil-based polyester polyol, where glycerol was used as a crosslinker and DBTL as a catalyst [18]. TDI and MDI were employed as isocyanates in this study. Radojčićetal., explored the ring-opening polymerization reaction of palm oil-based polyol to synthesize polyester PU with low hydroxyl content, with high molecular weight exhibiting a longer gel time [42].
Canola Oil
Canola oil has unsaturated double bonds in its fatty acid chains as well as an ester group, which allows it to be employed in the manufacturing of a wide range of important goods, such as polyols, owing to its high protein content and hydrophobic properties. Canola oil-based PU adhesives have excellent attributes such as equivalent lap shear strength, chemical resistance, and hot water resistance, implying that they have a lot of potential in particleboard manufacture. Li et al. [20] studied the adhesion attributes of sodium bisulfite (NaHSO3)-modified canola protein. Protein was extracted from canola meal through alkali solubilization and acid precipitation methods and was further modified with different concentrations of NaHSO3 (0–15 g/L). With the increase in the concentration of NaHSO3, the purity of canola protein was found to be decreased. The isolated wet protein was used as an adhesive.
Jatropha Oil
Jatropha oil is a non-edible, renewable oil with gum that can be utilized in adhesives, with excellent adhesion and improved mechanical characteristics. Aung et al. reported jatropha oil-based PU adhesive bearing epoxy groups which enhanced adhesion [19]. Gadhave et al. reported jatropha oil-based polyurethane adhesive via epoxidation reaction of hydroxylated polyols with 2,4-toluene diisocyanates [43]. Saalah et al., synthesized a renewable jatropha oil-based polyol by polymerization of jatropha oil with isophorone diisocyanate and dimethylol propionic acid; the product is widely used as a binder for wood and decorative coatings [22].
Soybean Oil
Soybean-based adhesives have mostly been utilized in the production of adhesives as well as wood composites such as particleboard and medium-density fibre board. Dai et al. synthesized Soy oil-based PU adhesives by reacting BIOH polyols X-0002 and IPDI via maintaining NCO/OH ratio 1:4 and examined the variations in physical and mechanical properties with hydroxyl functionalities in soy-based polyols in PU films [37].
2.1.3. Lignin
Lignin is generated as a by-product from the paper and pulp industry. The glass transition temperature, aromatic content of the final polymer, and thermal stability of adhesives can all be improved by including lignin into the polyurethane matrix as a polyol substitute. Due to its aromatic crosslinked structure, lignin is somewhat steady at high temperatures and works as a thermosetting material. Because of its aromatic composition, lignin acts as a hard segment and replaces the soft segment of PU, i.e., polyol, and boosts the mechanical strength, thermal characteristics, and composite material properties of blends, copolymers, and composite materials. Lignin incorporation into PU matrix leads to enhancement in its thermal stability, delamination, and resistance to abrasion [44]. Alinejad et al., prepared lignin-based polyurethane adhesives by polymerization of lignin with toluene -2,4-diisocyanate (TDI) and partial polyethylene glycol (PEG). Partial polyethylene glycol was used but, it was further replaced by lignin to avoid the hard and brittle property. The final product was used as wood adhesives. The amount of lignin incorporated enhanced the glass transition temperature and thermal stability of the adhesive [29].
2.1.4. Starch
Starch is widely being used in many industrial applications, such as the food industry, pharmaceutical industry, paper industry, and printing industry because of its inherent biodegradability, abundance, and renewability [45]. The main sources of starch are wheat, rice, potato, corn, etc. Two natural resources are potato starch and edible or non-edible plant-derived oils that can be employed to prepare PU adhesives. Starch glycosylation followed by oil transesterification to generate polyol can be used to make starch-based PU adhesives.
2.1.5. Polylactic Acid
Thermoplastic polyester polylactic acid is noted for its biodegradability. Lactate polyols are polyester polyols that include lactic acid units as a component and can be prepared from 100% bio-renewable material. Gadhave et al., prepared high functionality polyester polyols containing lactate polyols suitable for rigid cast polyurethane, having high bio-based content and that exhibit biodegradability [43].
2.1.6. Applications of Bio-Based PU Adhesive
Owing to the high stickiness on a variety of substrates such as metals, wood, plastics, glass, etc. These bio-based polyurethane adhesives have a wide range of industrial applications. The applications of bio-based polyurethane adhesive in assorted sectors are described below:
In Construction: PU adhesives are widely used in wood flooring, heavy-duty rubber flooring, and parquet bonding due to their great strength and resistance. PU adhesives are suitable for ceramic tiles, mirrors, bath surrounds, and concrete due to their ability to bond wet/frozen timber, as well as their high green strength and shear strength [27].
In Packaging: Snack food bags, printed films, and shopping bags commonly use one-component PU adhesives for adherence. In addition, for medical applications, a tailored two-component PU adhesive is used to adhere polyvinyl chloride to aluminium sheets. Hot melt PU adhesives are suited for wrapping applications due to their low viscosity and low application temperature.
Transportation: Polyurethane adhesives are used in a variety of industries, including automotive, trucks, and railways. Polyurethane adhesives are highly appreciated due to their short cure time and ability to generate strong flexible bonds [46].
Electronics: PU adhesives are good for bonding copper foil, electronic coil fabrication, surface fix conductive adhesion, and bonding die-stamped printed circuit boards due to their properties of curing at room temperature, endurance, and great resistance to thermal shocks and vibrations.
Footwear: The shoe industry requires PU adhesive to join waterproof insole materials and in the sole attachment because of its excellent water resistance, substantial heat resistance, flexibility in cold rapid settings, and good initial strength [47].
Biomedical applications: Tissue adhesives have gained popularity in recent decades, owing to an increase in the number of surgical procedures performed around the world. Depending on the adhesive purpose, these tissue adhesives are categorised into three basic types: (i) Hemostats, which prevent blood loss by interfering with the coagulation cascade, generally have weak mechanical characteristics and are employed in cases of blood loss owing to sutures tissue injury; (ii) glues that cling strongly to tissues and (iii) Sealants, which create a physical barrier to stop air/gas or blood seeping [48]; they usually provide mid-range tissue adhesion and are utilised in conjunction with sutures [34], and these systems, on the other hand, perform better in a dry atmosphere.
2.2. Polyurethane Coatings
PU coatings are used to enhance mechanical strength and to induce desired barrier properties in the components of aircraft, automotive, container ships, oil tankers, industrial machines, residential refrigerators, etc., [49]. In addition, these organic coatings shield the materials from intrusive environmental conditions such as humidity, biological deterioration, chemical and automated destruction, and radiation and impart colour and gloss [50]. Different types of PU coatings of industrial importance are discussed in the subsequent section.
High solids polyurethane coatings: High solids coatings have narrow molecular weight distribution and low solvent emissions; they have a suitable spray viscosity, which in combination with their low molecular weight, is responsible for their drying behaviour, without compromising the performance of the material [51].
Ultraviolet (UV) curable polyurethane coatings: PU curing polyurethane coatings have become popular in recent years. Polymerization and thermal curing processes, which involve solvents and catalysts, are used to fix traditional polyurethane coatings [52]. UV-curing technology is distinguished from thermal curing by the 5E abbreviation, which stands for Eco-Friendly, Economical, Efficiency, Enabling, and Energy Saving, making it “clean and green” [53]. Polyurethane acrylate-based UV-curable coatings are excellent in imparting abrasion resistance, impact resistance, scratch resistance, and optical transparency [53].
Hyperbranched polyurethane coatings: The three main types of dendritic polymers are dendrimers, hyperbranched polymers, and dendrigrafts. Because of their unique structural properties such as no entanglement and high surface functionalities, hyperbranched polymers and dendrimers emerged as an important class of polymeric materials in the recent years; they also have enhanced cross-link density, excellent reactivity, and reduced viscosity. Hyperbranched polymers, on the other hand, are preferred over dendrimers due to their one-step synthesis and fewer purification processes. Hyperbranched structures outperform linear structures in terms of characteristics [54]. Hyperbranched poly (ester amide) was also shown to be biodegradable, which might be considered a green advantage in advanced surface coating technology [55].
Water-borne polyurethane coatings: WPU material has received a lot of attention in the past few decades due to its many advantages, such as good flexibility, stiffness, impact and abrasion resistance, gloss, chemical resistance, lesser flammability, high adhesive strength, durability, low viscosity, easy cleaning and weather-ability, as well as zero or low volatile organic chemical compound emissions [56]. Because the viscosity of a WPU dispersion is independent of the polymer’s molecular weight, high solid content WPU dispersions with high-molecular-weight films can be produced with outstanding quality simply by physical drying [57]. A binary colloidal system in which PU particles are continually disseminated in aqueous solutions is known as PU dispersions. Due to ionic groups (PU ionomers) in the linear thermoplastic PU backbone, WPUs are dispersible in water. Polyurethanes, on the other hand, are not water-dispersible due to hydrophobic isocyanates (which also react with water) [58]. A PU ionomer is a copolymer with a PU backbone and repeat units that include pendant acid groups that are neutralised completely or partially to form salts. Internal emulsifiers include cationic-like quaternary ammonium groups, anionic-like carboxylated or sulfonated groups, and non-ionic groups such as polyols containing ethylene oxide end groups. Micelles are formed in water by cationic and anionic PU ionomers, respectively. The types of ionomers, isocyanates, and polyols utilised all have an impact on the performance of the resulting WPU dispersions.
UV curable WPU coatings: UV stable coatings cure quickly when exposed to ultraviolet light, resulting in outstanding performance. UV-curable waterborne polyurethane combines the advantages of UV equipment with those of aqueous coatings. UV-curable WPU coatings had been widely investigated because of their flexibility, non-toxic behaviour, chemical resistance, and remarkable mechanical properties. UV-WPU coatings, despite their many advantages, have worse solvent resistance and thermal stability than typical solvent-based polyurethane coatings, and the tensile strength is significantly reduced at temperatures above 80 °C. Due to their lower VOC emissions than solvent-based PU coatings, environmentally friendly waterborne polyurethane (WPU) coatings are widely employed [58]. The UV-cured WPU coatings are a significant class of green and environmentally friendly coatings with excellent mechanical qualities and a quick curing procedure. Due to numerous end groups, compact molecular structure, and reducing chain entanglement, hyperbranched polyurethanes (PUs) have intriguing features such as high solubility, reactivity, and good rheological behaviour.
Hyperbranched WPU coatings: Because of their distinct molecular architectures, such as compact molecular nature, numerous end groups, and decreased chain entanglement, hyperbranched polymers differ significantly from their linear counterparts. Branched-chain polymers have the most exciting properties, such as good rheological behaviour, strong reactivity, and solubility, and so have a wide range of applications [59]. The step-growth polycondensation technique is used to make polycarbonate, polyphenylene, polyesters, poly(ether ketone), polyamides, poly(4-chloromethylstyrene), polyurethanes, and other hyperbranched polymers. The number of generations, which is a statistical measure of the total number of monomers in a hyperbranched polymer, influences the compatibility of hyperbranched WPUs with nanofillers.
Nanocomposite WPU coatings: Waterborne surface coating materials with low volatile organic compounds (VOCs) are environmentally safe and, as a result, the most sought-after materials in today’s society. Waterborne polyurethane (WPU) coatings offer excellent stiffness and elasticity, but they have much lower mechanical strength and toughness than solvent-based coatings. Nano-sized inorganic fillers have been added to WPU dispersions to create nanostructured coatings, demonstrating that this is a potential method for increasing WPU properties. The manufactured WPU nanocomposites have a lot of potential as an environmentally friendly transparent surface coating material with low VOC content.
2.2.1. Vegetable Oil-Based Polyurethane Coatings
Polyurethane coatings based on vegetable oil have proven their worth by exhibiting significant properties such as abrasion and chemical resistance, remarkable toughness, corrosion resistance, low-temperature flexibility, and prospering industrial applications, as well as lowering or eliminating the use of volatile organic compounds (VOCs). The type of PU coating depends on the curing procedure and solvent employed. In addition to dispersing conventional polyurethane using petrochemical solvents, extensive research has been done on water-based PU extracts that use water as a solvent. Dispersions of waterborne PU have an inherent benefit over traditional in that they are environmentally friendly and safe to use [54]. PU treatment can occur at room temperature or below room temperature. UV curing is a type of thermal curing that uses UV radiation to provide the energy needed for the curing operation. To improve the properties of conventional PU coatings to fulfil the industrial norms, extensive investigation has been done on high-solid contents, nanocomposites, and hyperbranched coatings of PU, which are dependent on the respective properties and compatibility of distinct components [49]. Because it takes less time, uses less energy, and has a better curing action than other techniques, this method has proven to be more efficient than others. A sustainable alternative to mitigating failures from degradative corrosion is the appropriate utilization of domestically leftover resources such as vegetable oils (Figure 5).
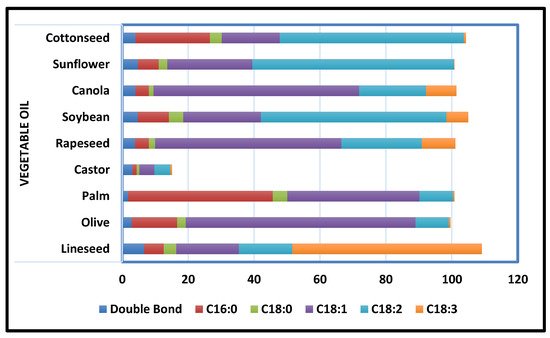
Figure 5. Common Vegetable Oils’ Degree of Unsaturation and Fatty Acid Composition [55].
2.2.2. PU Coatings Based on Cashew Nut Shell Liquid (CNSL)
In addition to vegetable oils, CNSL, an agricultural by-product of the cashew industry, has been utilized as binders for coatings material [60,61,62]. Meta substituted phenolic compounds identified in CNSL, a reddish-brown phenolic lipid, include 2-methyl cardol, cardol, cardinal, and anacardic acid. High vacuum distillation of CNSL yielded cardanol, a crucial precursor for a variety of oligomers and polymers [62,63]. For polymer curing, the meta-position of an unsaturated alkyl chain (C15H31-2n, n = 0–3) can be used as an autoxidizable site [64]. CNSL (cashew nut shell liquid) is a naturally occurring substituted phenol that can be used in place of phenol, with similar or superior outcomes. It is a low-cost and renewable material that finds use in Insecticidal, fungicidal, anti-termite, and therapeutic applications. In addition, it is also used as an additive in many plastic compositions. Cardanol-based polyols are considerably modified (the most common modification site is the phenolic hydroxyl group), resulting in autoxidizable bio-based polyurethane coatings with isocyanates [64]. All cardanol-based polyols have a 15-carbon side chain at the meta position, which may affect the polymers’ thermal and mechanical properties, as all changes are made to these unsaturation sites. Friction materials, cars, surface coatings, adhesives, laminates, rubber compounding, and a range of other applications use CNSL resins. The chemical structure of different cardanol-based polyols (CAP) from aromatic cardanol is depicted in Figure 6.
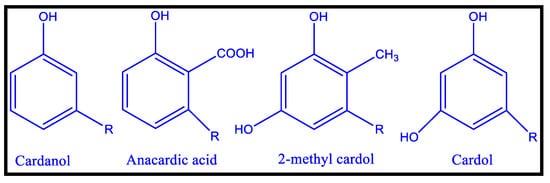
Figure 6. Chemical Structures of CNSL’s Major Components [63].
Because of the aromatic ring of cardanol, the PU backbone is particularly rigid, and the PU coating has a firm adherence to the metal, resulting in improved stiffness and hardness. Aside from CNLS-based polyol, CNLS-based cyclic carbonate (CC) was efficiently used as a precursor for non-isocyanate polyurethane preparation (NIPUs) [64]. For the synthesis of NIPU, di-glycidyl ether of cardanol (NC-514), a CNSL-derived product and epoxy formulation modification, was employed.
2.2.3. Polyurethane Coatings Based on Terpene
Terpene-based polyols with strong impact strength, water resistance, adhesion, flexibility, and pencil hardness can be crosslinked with poly-isocyanate to produce PU coatings (Figure 7). Terpenoids are good bio-based raw ingredients that have a high value in polymer applications [65].
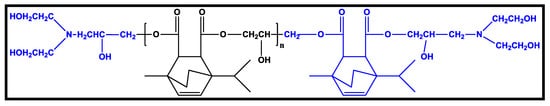
Figure 7. Chemical structure of polyols based on terpene [65].
2.2.4. Polyurethane Coatings Based on Eucalyptus Tar
Eucalyptus tar is a complicated combination primarily made up of phenols, particularly guaiacyl and syringyl derivatives [66]. A solid residue known as bio-pitch is created during the distillation of bio-oil, and it makes up a considerable component of the final product (approximately 50%). To make a tar-based varnish and a bio-pitch-based black paint, eucalyptus tar derivatives are used as a source of hydroxyl groups. Eucalyptus tar was distilled to produce heavy oil and bio-pitch, which were then mixed with castor oil to create a renewable polyol [67].
This entry is adapted from the peer-reviewed paper 10.3390/macromol2030019
This entry is offline, you can click here to edit this entry!
