Tetanus caused by the tetanus toxin (TT) is a fatal illness, which despite the existence of a vaccine, led to an estimated 34,684 deaths in 2019. TT is a neurotoxin produced by Clostridium tetani, a Gram-positive pathogenic bacterium, mainly found in soil and the gastrointestinal tracts of animals. TT induces the inhibition of neurotransmitter release, leading to spastic paralysis in a four-step process. First, TT binds to specific receptors, mainly composed of lipids and gangliosides, found at the neuromuscular junction (NMJ). Another receptor is reached by TT after these first bindings: a protein receptor responsible for its internalization (second step). This double receptor binding is responsible for the high affinity between TT and nerve cells. Third, TT is then transported into the cell body via axonal retrograde transport. In the last step, the proteolytic cleavage by TT of the VAMP/synaptobrevin, a neuronal substrate, leads to the inhibition of neurotransmitter release. All of these biological properties can be distinct associated parts of the TT structure. TT is a 150.7 kDa protein composed of a 52.4 kDa light chain and a 98.3 kDa heavy chain linked by a disulfide bond.
- tetanus toxin fragment C
- structure
- production
- uses
- vaccine
- neuronal protection
- CNS delivery
- immunogenicity
- carrier protein
- fusion protein
1. Tetanus Toxin Fragment C (TTFC) Properties and Uses: Central Nervous System (CNS) Delivery and Immunogenicity
In view of the numerous potential therapeutic applications of TTFC, especially in the fields of vaccination and neurology, different methods of production, characterization and evaluation were developed. An overview of TTFC by describing its numerous properties regarding immunology and neuroprotection will be offered, but also reflecting the different methods used for producing it (Figure 1).
Figure 1. Key points on TTFC.
1.1. TTFC Neurological Properties
1.1.1. Permeability and CNS Delivery
1.1.2. Intrinsic Neuronal Protection
1.1.3. Overview of the Uses of TTFC for Its Neurological Properties
| Medicinal Product |
Biological Interest | Administration and Dose | Experimental Model |
Observed Effects | Ref. |
|---|---|---|---|---|---|
| TTFC used alone | |||||
| TTFC | neuronal protection (ALS) |
Intramuscular 1 μg |
male and female SOD1-G93A mice |
|
[20] |
| TTFC | neuropsychiatric disorders (depression) |
intramuscular 20–60 μg/kg |
adult male Wistar-Kyoto rats |
|
[21] |
| TTFC | neuronal protection (spinal MN degeneration) |
direct spinal infusion (total amount of ~42 ng/rat) intramuscular (total amount of ~400 ng/rat) |
adult male Wistar rats |
|
[22] |
| TTFC | neuronal protection (PD) |
intraperitoneal 0.5 mg/kg |
male 8-week-old Sprague–Dawley rats |
|
[23] |
| TTFC | neuronal protection (AD, effect on learning and memory) |
medial septum (local administration) 100 ng |
adult male Wistar rats |
|
[24] |
| TTFC | neuronal protection (post-methamphetamine treatment) |
intramuscular 40 μg/kg |
adult male C57BL/6J mice |
|
[25] |
| TTFC | neuronal protection (restorative effect) |
intramuscular 20 µg/kg |
adult male Wistar rats |
|
[26] |
| Naked DNA encoding for TTFC |
neuronal protection (cerebral ischemia) |
intramuscular 200 µg |
adult male Mongolian gerbils |
|
[19] |
| Naked DNA encoding for TTFC |
neuronal protection (ALS disease) |
intramuscular 300 µg |
SOD1-G93A mice |
|
[12] |
| TTFC used as a fusion protein | |||||
| TTFC fused with rAAV8, CMV and eGFP | tracing study (connectivity map) |
hippocampal injection 1 µL |
adult male and female tdTomatoJ mice |
|
[27] |
| TTFC fused with GDNF |
neuronal protection (ALS disease) |
intramuscular 300 µg |
SODG93A mice |
|
[28] |
| TTFC fused with GFP |
study of neuronal network (study of nerve injury) |
/ | transgenic mice (NPY-Cre, ZWX) |
|
[29] |
| TTFC fused with IGF-1 |
neuronal protection(age related nerve alteration) |
intramuscular 10 µg |
old control FVB and DBA mice |
|
[30] |
| TTFC fused with GFP or β-galactosidase |
study of neuronal network (muscle specific spinal motor circuitry) |
intramuscular 10.57–19.2 µg/mL |
new born BalbC/J mice |
|
[31] |
| TTFC fused with SOD1 |
neuronal delivery (protein) |
intra- cerebroventricular |
adult male C57BL6 mice |
|
[7] |
| Other forms of TTFC (analog, complex, conjugate) | |||||
| 125I-TTFC | retrograde transport (spinal cord) |
intramuscular 10 µg of radiolabeled TTC |
transgenic mice (C57BL6, SOD193A) |
|
[32] |
| PEISH-based NP with HC | neuronal delivery (gene therapy) |
subcutaneous 150 μL of dispersion (conc. 7.5 µg pegylated HC per 2 µg of pDNA) |
male 4-month old Wistar rats |
|
[33] |
| Synthetic analog of TTFC, Tet1-peptide | neuronal delivery (small molecules) |
intramuscular 1 µL/g of body weight) |
young adult male heterozygous rats |
|
[11] |
| TTFC chemically coupled to GDNF | neuronal delivery (therapeutics) |
intramuscular 60–100 µg |
adult male mice |
|
[34] |
1.2. Immunological Properties
1.2.1. Immunological Properties against Tetanus
1.2.2. TTFC as a Fusion Protein: Enhancement of Immunogenicity
1.2.3. In Silico Design of Epitope-Based Vaccines
1.2.4. TTFC Uses for Its Immunological Properties
| Medicinal Product |
Biological Interest | Administration and Dose | Experimental Model |
Observed Effects | Ref. |
|---|---|---|---|---|---|
| TTFC used alone | |||||
| TTFC | tetanus antitoxin | Intramuscular 0.625–15 mg |
horses |
|
[70] |
| TTFC | vaccine (tetanus) |
/ | mAbs obtained after BALB/c mice immunization with TT |
|
[51] |
| 0.1 mg | BALB/c mice | ||||
| TTFC | vaccine (tetanus) |
transcutaneous 30 µg |
BALB/c mice |
|
[52] |
| TTFC used as fusion protein | |||||
| TTFC fused with S. aureus coagulase R domain | vaccine (S. aureus) |
intramuscular 30 µg of TTFC-CoaR |
BALB/c mice |
|
[71] |
| TTFC fused with several epitopes | cancer vaccine (HPV-induced cancer) |
subcutaneous 1.5 nmol of MEV (100 µL) |
C57BL/6 mice |
|
[69] |
| TTFC fused to flagellin |
mucosal vaccine (tetanus) |
intranasal 2.75 μg |
female BALB/c mice |
|
[72] |
| TTFC fused with DNA | cancer vaccine (multiple myeloma) |
intramuscular 6 times 1 mg fusion vaccine |
clinical trial—phase I 14 patients with multiple myeloma |
|
[73] |
| TTFC fused with Tem 1 cDNA | cancer vaccine (tumor vasculature) |
intramuscular 50 µg of plasmid in saline |
C57BL/6 and BALB/c mice |
|
[74] |
| TTFC domain fused with DNA (PSMA27–35) | cancer vaccine (prostate) |
intramuscular 5 times 400–3200 µg of fusion vaccine |
clinical trial—phase I/II 32 HLA-A2+ patients and 32 HLA-A2− control patients |
|
[75] |
| TTFC fused with naked DNA (VHCDR3109–116) | cancer vaccine (lymphoma) |
intramuscular 50 µg DNA plasmid |
male C3H/HeN mice |
|
[76] |
| TTFC fused with DNA | DNA vaccine (HPV 16 E6 and E7) |
intradermal tattoo vaccination 20 µg |
C57BL/6 mice |
|
[59] |
| TTFC fused with Cryptosporidium parvum antigens | vaccine (Cryptosporidium parvum) |
per os single dose 5 × 109 CFU |
female C57BL/6 and IL18-KO mice |
|
[77] |
| Other forms of TTFC (conjugate, bacteria) | |||||
| TTFC conjugated to pneumococcal polysaccharide |
vaccine (Pneumococcus) |
intraperitoneal 2 µg/mL of PS per vaccine |
female BALB/c mice |
|
[78] |
| TTFC and S. aureus surface protein A (SasA) | combined vaccine (tetanus and S. aureus) |
intraperitoneal 10 µg SasA + 10 µg TTFC |
female BALB/c mice |
|
[79] |
| TTFC conjugated to Her2 protein fragment |
cancer vaccine (Her2+ breast cancer) |
subcutaneous 50 µg of conjugate, 4 boosters of 25 µg |
female BALB-neuT mice |
|
[80] |
| TTFC conjugated to Burkholderia pseudomallei PS | vaccine (melioidosis) |
intraperitoneal 66 µg of conjugate per dose |
female BALB/c mice |
|
[81] |
| TTFC conjugate to Vibrio cholerae OPS | conjugate vaccine (cholera) |
intramuscular and intradermal 10 µg of OPS per animal (5:1 conjugate molar ratio OPS:TTFC) |
female Swiss- Webster mice |
|
[63] |
| Cytomegalovirus expressing TTFC | vaccine (tetanus) |
intraperitoneal 5 × 106 pfu |
age-matched female 129S1/SvlmJ/Cr mice |
|
[82] |
| Bacillus subtilis expressing TTFC |
vaccine (tetanus) |
sublingual and intranasal 1 × 109 cells of died TTFC-expressing B. subtilis |
weaned piglets |
|
[54] |
2. TTFC Production
2.1. Papain Digestion
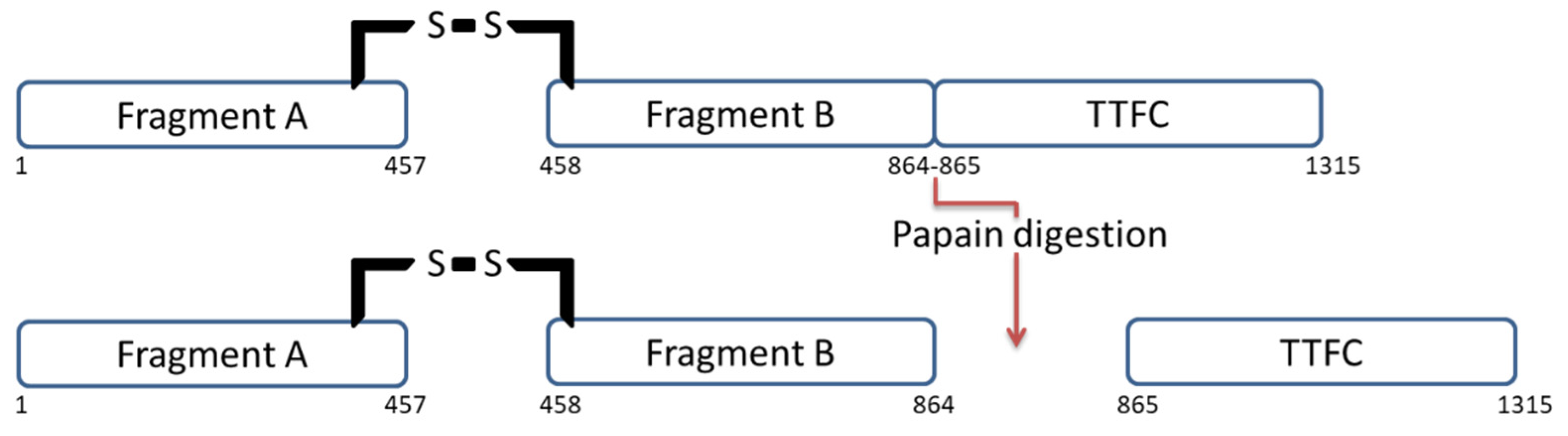 Figure 2. TT structure before and after papain digestion. Papain digests the protein by splitting it into two fragments: TTFC and fragment A-B.
Figure 2. TT structure before and after papain digestion. Papain digests the protein by splitting it into two fragments: TTFC and fragment A-B.2.2. TTFC Production in Recombinant Systems
2.2.1. Escherichia coli as a Host for TTFC Production
| Expression Conditions |
Fairweather et al. 1986 [90] |
Makkof et al. 1989 [88] |
Makkof et al. 1989 [95] |
Halpern et al. 1990 [89] |
Ribas et al. 2000 [96] |
Motamedi et al. 2011 [93] |
Yu et al. 2011 [92] |
Yu et al. 2011 [94] |
Yousefi et al. 2013 [91] |
Aghayipour et al. 2018 [97] |
|---|---|---|---|---|---|---|---|---|---|---|
| host | DH1 | E. coli | E. coli | DH5α | BL21 | DH5α | BL21 | BL21 | BL21 | BL21pLys |
| TTFC DNA origin | C. tetani | synthetic for end of TTFC |
synthetic (optimized codons for TTFC) |
C. tetani | C. tetani | C. tetani | synthetic | synthetic (optimized AT: 72.50% to 52.47%) |
C. tetani | C. tetani |
| recombinant protein | TrpE-TTFC (trpE: anthranilate synthetase) |
1: met-3AA INFγ-TTFB(537–864)-TTFC(865–1315) 2: met-TTFC |
met-TTFC | fusion with 8AA from vector and 9AA from fragment B | 112AA Trx-45AA TTFC- His-tag |
MBP-TTFC (MBP:maltose binding protein) |
Trx-TTFC-6His tag | no tag | Cterm of TTFC (25 kDa)-6His tag | 6His-tagged fusion protein |
| plasmid | pWRL507 | pTET-Tact1 pTET-Tact2 |
pTET-Tact2 | pTTQ8 | pET32a | pMalc2x | pTIG-Trx | pET32a+ | pET28b+ | pET28a pET22a |
| promotor | trpE | tac (derived from trp and lac UV5) |
tac | tac | T7 | tac | T7 | T7 | T7 | T7 |
| inducer | indoylacrylic acid |
IPTG 60 µg/mL |
IPTG | IPTG 0.67 mM |
IPTG 1 mM |
IPTG | IPTG 0.4 mM |
IPTG 0.2 mM |
IPTG 1 mM |
IPTG (optimized protocol) |
| quantity | low amount of fusion protein /trpE protein |
2: 12 mg TTFC/L (3–4% TPC) |
11–14% TPC (with optimized promotor) |
1 mg/L (0.5% TPC) |
35 mg/L | un-specified | 15–30% TPC (20–35 mg/mL after purification) |
333 mg/L 42 L fermentor (46% TPC) |
35% TCP | pET28a: 38 mg/mL pET22a: 32 mg/mL |
| solubility | soluble | 1: low solubilty 2: soluble |
soluble | soluble | soluble | soluble | soluble | soluble | soluble | soluble |
2.2.2. TTFC Expression and Delivery in Other Bacterial Host Strains
2.2.3. Recombinant TTFC Expression in Yeast and Plant Cells
3. Conclusion
This entry is adapted from the peer-reviewed paper 10.3390/ph15060756
References
- Herreros, J.; Lalli, G.; Schiavo, G. C-terminal half of tetanus toxin fragment C is sufficient for neuronal binding and interaction with a putative protein receptor. Biochem. J. 2000, 347 Pt 1, 199–204.
- Vajn, K.; Viljetić, B.; Degmečić, I.V.; Schnaar, R.L.; Heffer, M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE 2013, 8, e75720.
- Toivonen, J.M.; Oliván, S.; Osta, R. Tetanus toxin C-fragment: The courier and the cure? Toxins 2010, 2, 2622–2644.
- Calvo, A.C.; Oliván, S.; Manzano, R.; Zaragoza, P.; Aguilera, J.; Osta, R. Fragment C of tetanus toxin: New insights into its neuronal signaling pathway. Int. J. Mol. Sci. 2012, 13, 6883–6901.
- Gramlich, P.A.; Remington, M.P.; Amin, J.; Betenbaugh, M.J.; Fishman, P.S. Tat-tetanus toxin fragment C: A novel protein delivery vector and its use with photochemical internalization. J. Drug Target. 2013, 21, 662–674.
- Francis, J.W.; Bastia, E.; Matthews, C.C.; Parks, D.A.; Schwarzschild, M.A.; Brown, R.H.; Fishman, P.S. Tetanus toxin fragment C as a vector to enhance delivery of proteins to the CNS. Brain Res. 2004, 1011, 7–13.
- Benn, S.C.; Ay, I.; Bastia, E.; Chian, R.-J.; Celia, S.A.; Pepinsky, R.B.; Fishman, P.S.; Brown, R.H.; Francis, J.W. Tetanus toxin fragment C fusion facilitates protein delivery to CNS neurons from cerebrospinal fluid in mice. J. Neurochem. 2005, 95, 1118–1131.
- Bordet, T.; Castelnau-Ptakhine, L.; Fauchereau, F.; Friocourt, G.; Kahn, A.; Haase, G. Neuronal targeting of cardiotrophin-1 by coupling with tetanus toxin C fragment. Mol. Cell. Neurosci. 2001, 17, 842–854.
- Kissa, K.; Mordelet, E.; Soudais, C.; Kremer, E.J.; Demeneix, B.A.; Brûlet, P.; Coen, L. In vivo neuronal tracing with GFP-TTC gene delivery. Mol. Cell. Neurosci. 2002, 20, 627–637.
- Miana-Mena, F.J.; Roux, S.; Benichou, J.-C.; Osta, R.; Brûlet, P. Neuronal activity-dependent membrane traffic at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2002, 99, 3234–3239.
- Kassa, R.; Monterroso, V.; David, L.L.; Tshala-Katumbay, D. Diagnostic and therapeutic potential of tetanus toxin-derivatives in neurological diseases. J. Mol. Neurosci. 2013, 51, 788–791.
- Moreno-Igoa, M.; Calvo, A.C.; Penas, C.; Manzano, R.; Oliván, S.; Muñoz, M.J.; Mancuso, R.; Zaragoza, P.; Aguilera, J.; Navarro, X.; et al. Fragment C of tetanus toxin, more than a carrier. Novel perspectives in non-viral ALS gene therapy. J. Mol. Med. 2010, 88, 297–308.
- Mendieta, L.; Bautista, E.; Sánchez, A.; Guevara, J.; Herrando-Grabulosa, M.; Moran, J.; Martínez, R.; Aguilera, J.; Limón, I.D. The C-terminal domain of the heavy chain of tetanus toxin given by intramuscular injection causes neuroprotection and improves the motor behavior in rats treated with 6-hydroxydopamine. Neurosci. Res. 2012, 74, 156–167.
- Chaïb-Oukadour, I.; Gil, C.; Rodríguez-Alvarez, J.; Ortega, A.; Aguilera, J. Tetanus toxin HC fragment reduces neuronal MPP+ toxicity. Mol. Cell. Neurosci. 2009, 41, 297–303.
- Chaïb-Oukadour, I.; Gil, C.; Aguilera, J. The C-terminal domain of the heavy chain of tetanus toxin rescues cerebellar granule neurones from apoptotic death: Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. J. Neurochem. 2004, 90, 1227–1236.
- Mendieta, L.; Venegas, B.; Moreno, N.; Patricio, A.; Martínez, I.; Aguilera, J.; Limón, I.D. The carboxyl-terminal domain of the heavy chain of tetanus toxin prevents dopaminergic degeneration and improves motor behavior in rats with striatal MPP+-lesions. Neurosci. Res. 2009, 65, 98–106.
- Gil, C.; Chaib-Oukadour, I.; Aguilera, J. C-terminal fragment of tetanus toxin heavy chain activates Akt and MEK/ERK signalling pathways in a Trk receptor-dependent manner in cultured cortical neurons. Biochem. J. 2003, 373 Pt 2, 613–620.
- Cubí, R.; Candalija, A.; Ortega, A.; Gil, C.; Aguilera, J. Tetanus toxin Hc fragment induces the formation of ceramide platforms and protects neuronal cells against oxidative stress. PLoS ONE 2013, 8, e68055.
- Radenovic, L.; Selakovic, V.; Olivan, S.; Calvo, A.C.; Rando, A.; Janac, B.; Osta, R. Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res. Bull. 2014, 101, 37–44.
- Moreno-Martinez, L.; de la Torre, M.; Muñoz, M.J.; Zaragoza, P.; Aguilera, J.; Calvo, A.C.; Osta, R. Neuroprotective fragment C of tetanus toxin modulates IL-6 in an ALS mouse model. Toxins 2020, 12, 330.
- Getachew, B.; Mendieta, L.; Csoka, A.B.; Aguilera, J.; Tizabi, Y. Antidepressant effects of C-terminal domain of the heavy chain of tetanus toxin in a rat model of depression. Behav. Brain Res. 2019, 370, 111968.
- Netzahualcoyotzi, C.; Tapia, R. Tetanus toxin C-fragment protects against excitotoxic spinal motoneuron degeneration in vivo. Sci. Rep. 2018, 8, 16584.
- Moreno-Galarza, N.; Mendieta, L.; Palafox-Sánchez, V.; Herrando-Grabulosa, M.; Gil, C.; Limón, D.I.; Aguilera, J. Peripheral administration of tetanus toxin Hc fragment prevents MPP+ toxicity In vivo. Neurotox. Res. 2018, 34, 47–61.
- Patricio-Martínez, A.; Mendieta, L.; Martínez, I.; Aguilera, J.; Limón, I.D. The recombinant C-terminal fragment of tetanus toxin protects against cholinotoxicity by intraseptal injection of β-amyloid peptide (25–35) in rats. Neuroscience 2016, 315, 18–30.
- Mendieta, L.; Granado, N.; Aguilera, J.; Tizabi, Y.; Moratalla, R. Fragment C domain of tetanus toxin mitigates methamphetamine neurotoxicity and its motor consequences in mice. Int. J. Neuropsychopharmacol. 2016, 19, pyw021.
- Sánchez-González, A.; Mendieta, L.; Palafox, V.; Candalija, A.; Luna, F.; Aguilera, J.; Limón, I.D. The restorative effect of intramuscular injection of tetanus toxin C-fragment in hemiparkinsonian rats. Neurosci. Res. 2014, 84, 1–9.
- Bohne, P.; Schwarz, M.K.; Herlitze, S.; Mark, M.D. A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front. Neural Circuits 2019, 13, 51.
- Moreno-Igoa, M.; Calvo, A.C.; Jesús, C.; Muñoz, M.J.; Zaragoza, P.; Rosario, O. Non-viral gene delivery of the GDNF, either alone or fused to the C-fragment of tetanus toxin protein, prolongs survival in a mouse ALS model. Restor. Neurol. Neurosci. 2012, 30, 69–80.
- Bráz, J.M.; Basbaum, A.I. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience 2009, 163, 1220–1232.
- Payne, A.M.; Messi, M.L.; Zheng, Z.; Delbono, O. Motor neuron targeting of IGF-1 attenuates age-related external Ca2+-dependent skeletal muscle contraction in senescent mice. Exp. Gerontol. 2007, 42, 309–319.
- Perreault, M.C.; Bernier, A.P.; Renaud, J.S.; Roux, S.; Glover, J.C. C fragment of tetanus toxin hybrid proteins evaluated for muscle-specific transsynaptic mapping of spinal motor circuitry in the newborn mouse. Neuroscience 2006, 141, 803–816.
- Lee, P.J.; Kennedy, Z.; Wang, Y.; Lu, Y.; Cefaliello, C.; Uyan, Ö.; Song, C.Q.; da Cruz Godinho, B.M.; Xu, Z.; Rusckowski, M.; et al. Imaging net retrograde axonal transport in vivo: A physiological biomarker. Ann. Neurol. 2022, 91, 716–729.
- Lopes, C.D.; Oliveira, H.; Estevão, I.; Pires, L.R.; Pêgo, A.P. In vivo targeted gene delivery to peripheral neurons mediated by neurotropic poly(ethylene imine)-based nanoparticles. Int. J. Nanomed. 2016, 11, 2675–2683.
- Larsen, K.E.; Benn, S.C.; Ay, I.; Chian, R.-J.; Celia, S.A.; Remington, M.P.; Bejarano, M.; Liu, M.; Ross, J.; Carmillo, P.; et al. A glial cell line-derived neurotrophic factor (GDNF): Tetanus toxin fragment C protein conjugate improves delivery of GDNF to spinal cord motor neurons in mice. Brain Res. 2006, 1120, 1–12.
- Reece, J.C.; Geysen, H.M.; Rodda, S.J. Mapping the major human T helper epitopes of tetanus toxin. The emerging picture. J. Immunol. 1993, 151, 6175–6184.
- Diethelm-Okita, B.M.; Okita, D.K.; Banaszak, L.; Conti-Fine, B.M. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 2000, 181, 1001–1009.
- Diethelm-Okita, B.M.; Raju, R.; Okita, D.K.; Contl-Fine, B.M. Epitope repertoire of human CD4+ T cells on tetanus toxin: Identification of immunodominant sequence segments. J. Infect. Dis. 1997, 175, 382–391.
- Valmori, D.; Pessi, A.; Bianchi, E.; Corradin, G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J. Immunol. 1992, 149, 717–721.
- Panina-Bordignon, P.; Tan, A.; Termijtelen, A.; Demotz, S.; Corradin, G.; Lanzavecchia, A. Universally immunogenic T cell epitopes: Promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur. J. Immunol. 1989, 19, 2237–2242.
- Biradhar, N.; Nimmagadda, S.V.; Aavula, S.M.; Parthasarathy, S.; Sula, S.; Maithal, K. Identification and characterization of novel binding epitope of tetanus toxoid by phage display peptide library. Curr. Trends Biotechnol. Pharm. 2015, 9, 49–58.
- Nezafat, N.; Ghasemi, Y.; Javadi, G.; Khoshnoud, M.J.; Omidinia, E. A novel multi-epitope peptide vaccine against cancer: An in silico approach. J. Theor. Biol. 2014, 349, 121–134.
- James, E.A.; Bui, D.; Berger, J.; Huston, L.; Roti, M.; Kwok, W.W. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int. Immunol. 2007, 19, 1291–1301.
- Ghafari-Khamene, M.; Torabi-Goudarzi, S.; Hosseini, M.; Haji-Fatahaliha, M.; Sadreddini, S.; Seyfi-Najmi, M.; Majidi, J.; Yousefi, M. Response of human T cells to tetanus neurotoxin HCC sub-domain: T cell cytokine production and activation marker induced by HCC. Iran. J. Allergy Asthma Immunol. 2015, 14, 519–525.
- Yousefi, M.; Younesi, V.; Bayat, A.A.; Jadidi-Niaragh, F.; Abbasi, E.; Razavi, A.; Khosravi-Eghbal, R.; Asgarin-Omran, H.; Shokri, F. Comparative human and mouse antibody responses against tetanus toxin at clonal level. J. Immunotoxicol. 2016, 13, 243–248.
- Volk, W.A.; Bizzini, B.; Snyder, R.M.; Bernhard, E.; Wagner, R.R. Neutralization of tetanus toxin by distinct monoclonal antibodies. Binding to multiple epitopes on the toxin molecule. Infect. Immun. 1984, 45, 604–609.
- Matsuda, M.; Kamei, M.; Sugimoto, N.; Ma, Y.; Hashizume, S. Characteristics of toxin-neutralization by anti-tetanus human monoclonal antibodies directed against the three functional domains , and of the tetanus toxin molecule and a reliable method for evaluating the protective effects of monoclonal antibodies. Eur. J. Epidemiol. 1992, 8, 1–8.
- Wang, Y.; Wu, C.; Yu, J.; Lin, S.; Liu, T.; Zan, L.; Li, N.; Hong, P.; Wang, X.; Jia, Z.; et al. Structural basis of tetanus toxin neutralization by native human monoclonal antibodies. Cell Rep. 2021, 35, 109070.
- Lukić, I.; Marinković, E.; Filipović, A.; Krnjaja, O.; Kosanović, D.; Inić-Kanada, A.; Stojanović, M. Key protection factors against tetanus: Anti-tetanus toxin antibody affinity and its ability to prevent tetanus toxin—Ganglioside interaction. Toxicon 2015, 103, 135–144.
- Ghotloo, S.; Golsaz-Shirazi, F.; Amiri, M.M.; Jeddi-Tehrani, M.; Shokri, F. Epitope mapping of tetanus toxin by monoclonal antibodies: Implication for immunotherapy and vaccine design. Neurotox. Res. 2020, 37, 239–249.
- Fairweather, N.F.; Lyness, V.A.; Maskell, D.J. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect. Immun. 1987, 55, 2541–2545.
- Luo, P.; Qin, L.; Mao, X.; Chen, L.; Yu, S.; Li, Q.; Liu, W.; Zhang, W.; Gu, J.; Zou, Q. Identification of a novel linear epitope in tetanus toxin recognized by a protective monoclonal antibody: Implications for vaccine design. Vaccine 2012, 30, 6449–6455.
- Johnston, L.; Mawas, F.; Tierney, R.; Qazi, O.; Fairweather, N.F.; Sesardic, D. Transcutaneous delivery of tetanus toxin Hc fragment induces superior tetanus toxin neutralizing antibody response compared to tetanus toxoid. Hum. Vaccin. 2009, 5, 230–236.
- Qazi, O.; Sesardic, D.; Tierney, R.; Soderback, Z.; Crane, D.; Bolgiano, B.; Fairweather, N.F. Reduction of the ganglioside binding activity of the tetanus toxin HC fragment destroys immunogenicity: Implications for development of novel tetanus vaccines. Infect. Immun. 2006, 74, 4884–4891.
- Amuguni, J.H.; Lee, S.; Kerstein, K.O.; Brown, D.W.; Belitsky, B.R.; Herrmann, J.E.; Keusch, G.T.; Sonenshein, A.L.; Tzipori, S. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine 2011, 29, 4778–4784.
- Fishman, P.S.; Matthews, C.C.; Parks, D.A.; Box, M.; Fairweather, N.F. Immunization does not interfere with uptake and transport by motor neurons of the binding fragment of tetanus toxin. J. Neurosci. Res. 2006, 83, 1540–1543.
- Ramakrishnan, G.; Wright, M.; Alam, M.; Naylor, C.; Kabir, M.; Zerin, A.; Ferdous, T.; Pedersen, K.; Hennig, B.J.; Donowitz, J.R.; et al. Rapid assessment of tetanus vaccine-induced immunity in Bangladesh and the Gambia. Diagn. Microbiol. Infect. Dis. 2017, 87, 272–274.
- Leitner, W.W.; Baker, M.C.; Berenberg, T.L.; Lu, M.C.; Yannie, P.J.; Udey, M.C. Enhancement of DNA tumor vaccine efficacy by gene gun-mediated codelivery of threshold amounts of plasmid-encoded helper antigen. Blood 2009, 113, 37–45.
- Henken, F.E.; Oosterhuis, K.; Öhlschläger, P.; Bosch, L.; Hooijberg, E.; Haanen, J.B.A.G.; Steenbergen, R.D.M. Preclinical safety evaluation of DNA vaccines encoding modified HPV16 E6 and E7. Vaccine 2012, 30, 4259–4266.
- Oosterhuis, K.; Öhlschläger, P.; Van den Berg, J.H.; Teebs, M.; Gomez, R.; Schumacher, T.N.; Haanen, J.B. Preclinical development of highly effective and safe DNA vaccines directed against HPV 16 E6 and E7. Int. J. Cancer 2011, 129, 397–406.
- Jahouh, F.; Xu, P.; Vann, W.F.; Kováč, P.; Banoub, J.H. Mapping the glycation sites in the neoglycoconjugate from hexasaccharide antigen of Vibrio cholerae, serotype Ogawa and the recombinant tetanus toxin C-fragment carrier: Glycations sites of rTT-Hc neoglycoconjugates. J. Mass Spectrom. 2013, 48, 1083–1090.
- McCarthy, P.C.; Saksena, R.; Peterson, D.C.; Lee, C.H.; An, Y.; Cipollo, J.F.; Vann, W.F. Chemoenzymatic synthesis of immunogenic meningococcal group C polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj. J. 2013, 30, 857–870.
- Scott, A.E.; Ngugi, S.A.; Laws, T.R.; Corser, D.; Lonsdale, C.L.; D’Elia, R.V.; Titball, R.W.; Williamson, E.D.; Atkins, T.P.; Prior, J.L. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J. Immunol. Res. 2014, 2014, 392170.
- Sayeed, M.A.; Bufano, M.K.; Xu, P.; Eckhoff, G.; Charles, R.C.; Alam, M.M.; Sultana, T.; Rashu, M.R.; Berger, A.; Gonzalez-Escobedo, G.; et al. A Cholera conjugate vaccine containing O-specific polysaccharide (OSP) of V. cholerae O1 Inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHc) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Negl. Trop. Dis. 2015, 9, e0003881.
- Xu, P.; Kelly, M.; Vann, W.F.; Qadri, F.; Ryan, E.T.; Kováč, P. Conjugate vaccines from bacterial antigens by squaric acid chemistry: A closer look. ChemBioChem 2017, 18, 799–815.
- Karkhah, A.; Saadi, M.; Nouri, H.R. In silico analyses of heat shock protein 60 and calreticulin to designing a novel vaccine shifting immune response toward T helper 2 in atherosclerosis. Comput. Biol. Chem. 2017, 67, 244–254.
- Saadi, M.; Karkhah, A.; Nouri, H.R. Development of a multi-epitope peptide vaccine inducing robust T cell responses against brucellosis using immunoinformatics based approaches. Infect. Genet. Evol. 2017, 51, 227–234.
- Validi, M.; Karkhah, A.; Prajapati, V.K.; Nouri, H.R. Immuno-informatics based approaches to design a novel multi epitope-based vaccine for immune response reinforcement against leptospirosis. Mol. Immunol. 2018, 104, 128–138.
- Safavi, A.; Kefayat, A.; Abiri, A.; Mahdevar, E.; Behnia, A.H.; Ghahremani, F. In silico analysis of transmembrane protein 31 (TMEM31) antigen to design novel multiepitope peptide and DNA cancer vaccines against melanoma. Mol. Immunol. 2019, 112, 93–102.
- Nezafat, N.; Sadraeian, M.; Rahbar, M.R.; Khoshnoud, M.J.; Mohkam, M.; Gholami, A.; Banihashemi, M.; Ghasemi, Y. Production of a novel multi-epitope peptide vaccine for cancer immunotherapy in TC-1 tumor-bearing mice. Biologicals 2015, 43, 11–17.
- Yu, R.; Ji, C.; Xu, J.; Wang, D.; Fang, T.; Jing, Y.; Kwang-Fu Shen, C.; Chen, W. The immunogenicity of the C fragment of tetanus neurotoxin in production of tetanus antitoxin. Biomed. Res. Int. 2018, 2018, 6057348.
- Qian, M.; Zhao, T.; Li, R.; Yang, Q.; Yu, R.; Yin, Y.; Zai, X.; Li, Y.; Zhang, J.; Xu, J.; et al. Targeting the R domain of coagulase by active vaccination protects mice against lethal Staphylococcus aureus infection. Microbes Infect. 2019, 21, 163–169.
- Lee, S.E.; Nguyen, C.T.; Kim, S.Y.; Thi, T.N.; Rhee, J.H. Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine. Clin. Exp. Vaccine Res. 2015, 4, 59–67.
- McCann, K.J.; Godeseth, R.; Chudley, L.; Mander, A.; Di Genova, G.; Lloyd-Evans, P.; Kerr, J.P.; Malykh, V.B.; Jenner, M.W.; Orchard, K.H.; et al. Idiotypic DNA vaccination for the treatment of multiple myeloma: Safety and immunogenicity in a phase I clinical study. Cancer Immunol. Immunother. 2015, 64, 1021–1032.
- Facciponte, J.G.; Ugel, S.; De Sanctis, F.; Li, C.; Wang, L.; Nair, G.; Sehgal, S.; Raj, A.; Matthaiou, E.; Coukos, G.; et al. Tumor endothelial marker 1–specific DNA vaccination targets tumor vasculature. J. Clin. Investig. 2014, 124, 1497–1511.
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170.
- Iurescia, S.; Fioretti, D.; Pierimarchi, P.; Signori, E.; Zonfrillo, M.; Tonon, G.; Fazio, V.M.; Rinaldi, M. Genetic immunization with CDR3-based fusion vaccine confers protection and long-term tumor-free survival in a mouse model of lymphoma. J. Biomed. Biotechnol. 2010, 2010, 316069.
- Benitez, A.J.; McNair, N.; Mead, J.R. Oral immunization with attenuated Salmonella enterica serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin. Vaccine Immunol. 2009, 16, 1272–1278.
- Yu, R.; Xu, J.; Hu, T.; Chen, W. The pneumococcal polysaccharide-tetanus toxin native C-fragment conjugate vaccine: The carrier effect and immunogenicity. Mediators Inflamm. 2020, 2020, e9596129.
- Yang, Y.; Yu, R.; Yang, X.; Liu, S.; Fang, T.; Song, X.; Hou, L.; Yu, C.; Xu, J.; Fu, L.; et al. Protection against Staphylococcus aureus and tetanus infections by a combined vaccine containing SasA and TeNT-Hc in mice. Mol. Med. Rep. 2017, 15, 2369–2373.
- Chotprakaikiat, W.; Allen, A.; Bui-Minh, D.; Harden, E.; Jobsri, J.; Cavallo, F.; Gleba, Y.; Stevenson, F.K.; Ottensmeier, C.; Klimyuk, V.; et al. A plant-expressed conjugate vaccine breaks CD4(+) tolerance and induces potent immunity against metastatic Her2(+) breast cancer. Oncoimmunology 2016, 5, e1166323.
- Scott, A.E.; Christ, W.J.; George, A.J.; Stokes, M.G.; Lohman, G.J.; Guo, Y.; Jones, M.; Titball, R.W.; Atkins, T.P.; Campbell, A.S.; et al. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjug. Chem. 2016, 27, 1435–1446.
- Tierney, R.; Nakai, T.; Parkins, C.J.; Caposio, P.; Fairweather, N.F.; Sesardic, D.; Jarvis, M.A. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine 2012, 30, 3047–3052.
- Helting, T.; Zwister, O. Enzymatic breakdown of tetanus toxin. Biochem. Biophys. Res. Commun. 1974, 57, 1263–1270.
- Murzello, K.; Kaundinya, J.O.; Dandekar, S. Simplified method for purification of C-fragment from tetanus toxin and toxoid by enzymatic fragmentation and chromatography. Indo Am. J. Pharm. Res. 2014, 4, 4060–4066.
- Weller, U.; Dauzenroth, M.E.; Meyer zu Reindorf, D.; Habermann, E. Chains and fragments of tetanus toxin. Separation, reassociation and pharmacological properties. Eur. J. Biochem. 1989, 182, 649–656.
- Neubauer, V.; Helting, T.B. Structure of tetanus toxin: The arrangement of papain digestion products within the heavy chain-light chain framework of extracellular toxin. Biochim. Biophys. Acta 1981, 668, 141–148.
- Helting, T.B.; Zwisler, O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J. Biol. Chem. 1977, 252, 187–193.
- Makoff, A.J.; Ballantine, S.P.; Smallwood, A.E.; Fairweather, N.F. Expression of tetanus toxin fragment C in E. coli: Its purification and potential use as a vaccine. Nat. Biotechnol. 1989, 7, 1043–1046.
- Halpern, J.L.; Habig, W.H.; Neale, E.A.; Stibitz, S. Cloning and expression of functional fragment C of tetanus toxin. Infect. Immun. 1990, 58, 1004–1009.
- Fairweather, N.F.; Lyness, V.A.; Pickard, D.J.; Allen, G.; Thomson, R.O. Cloning, nucleotide sequencing, and expression of tetanus toxin fragment C in Escherichia coli. J. Bacteriol. 1986, 165, 21–27.
- Yousefi, M.; Khosravi-Eghbal, R.; Hemmati, A.; Shokri, F. Production and characterization of recombinant light chain and carboxyterminal heavy chain fragments of tetanus toxin. Avicenna J. Med. Biotechnol. 2013, 5, 220–226.
- Yu, Y.Z.; Gong, Z.W.; Ma, Y.; Zhang, S.M.; Zhu, H.Q.; Wang, W.B.; Du, Y.; Wang, S.; Yu, W.Y.; Sun, Z.W. Co-expression of tetanus toxin fragment C in Escherichia coli with thioredoxin and its evaluation as an effective subunit vaccine candidate. Vaccine 2011, 29, 5978–5985.
- Motamedi, H.; Seyfiabad Shapouri, M.R.; Ghorbanpour Najafabadi, M.; Arefzadeh, N. Cloning and expression of tetanus toxin C fragment (Fc) in prokaryotic vector for constructing recombinant protein based vaccine for tetanus. Iran. J. Vet. Res. 2011, 12, 107–112.
- Yu, R.; Hou, L.; Yu, C.; Liu, S.; Ren, J.; Fang, T.; Zhang, X.; Chen, W. Enhanced expression of soluble recombinant tetanus neurotoxin Hc in Escherichia coli as a tetanus vaccine candidate. Immunobiology 2011, 216, 485–490.
- Makoff, A.J.; Romanos, M.A.; Oxer, M.D.; Fairweather, N.F.; Ballantine, S.P. Expression of tetanus toxin fragment C in E. coli: High level expression by removing rare codons. Nucleic Acids Res. 1989, 17, 10191–10202.
- Ribas, A.V.; Ho, P.L.; Tanizaki, M.M.; Raw, I.; Nascimento, A.L.T.O. High-level expression of tetanus toxin fragment C–thioredoxin fusion protein in Escherichia coli. Biotechnol. Appl. Biochem. 2000, 31, 91–94.
- Aghayipour, K.; Teymourpour, R. High-level expression of tetanus toxin fragment C in Escherichia coli. Arch. Razi Inst. 2018, 73, 27–38.
- Koopaei, N.N.; Khadiv-Parsi, P.; Khoshayand, M.R.; Mazlomi, M.A.; Kebriaeezadeh, A.; Moloudian, H.; Solhi, R.; Aminian, M. Optimization of rPDT fusion protein expression by Escherichia coli in pilot scale fermentation: A statistical experimental design approach. AMB Express 2018, 8, 135.
- Zhang, X.; Betterle, N.; Hidalgo Martinez, D.; Melis, A. Recombinant protein stability in cyanobacteria. ACS Synth. Biol. 2021, 10, 810–825.
- Hidalgo Martinez, D.; Betterle, N.; Melis, A. Phycocyanin fusion constructs for heterologous protein expression accumulate as functional heterohexameric complexes in cyanobacteria. ACS Synth. Biol. 2022, 11, 1152–1166.
- Levine, M.M. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol. 2010, 8, 129.
- Maassen, C.B.; Laman, J.D.; den Bak-Glashouwer, M.J.; Tielen, F.J.; van Holten-Neelen, J.C.; Hoogteijling, L.; Antonissen, C.; Leer, R.J.; Pouwels, P.H.; Boersma, W.J.; et al. Instruments for oral disease-intervention strategies: Recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 1999, 17, 2117–2128.
- Reveneau, N.; Geoffroy, M.C.; Locht, C.; Chagnaud, P.; Mercenier, A. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 2002, 20, 1769–1777.
- Robinson, K.; Chamberlain, L.M.; Schofield, K.M.; Wells, J.M.; Le Page, R.W. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 1997, 15, 653–657.
- Grangette, C.; Muller-Alouf, H.; Hols, P.; Goudercourt, D.; Delcour, J.; Turneer, M.; Mercenier, A. Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria. Infect. Immun. 2004, 72, 2731–2737.
- Robinson, K.; Chamberlain, L.M.; Lopez, M.C.; Rush, C.M.; Marcotte, H.; Le Page, R.W.F.; Wells, J.M. Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C. Infect. Immun. 2004, 72, 2753–2761.
- Grangette, C.; Müller-Alouf, H.; Geoffroy, M.; Goudercourt, D.; Turneer, M.; Mercenier, A. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: Impact of strain viability and in vivo persistence. Vaccine 2002, 20, 3304–3309.
- Shaw, D.M.; Gaerthé, B.; Leer, R.J.; Van Der Stap, J.G.M.M.; Smittenaar, C.; Heijne Den Bak-Glashouwer, M.J.; Thole, J.E.R.; Tielen, F.J.; Pouwels, P.H.; Havenith, C.E.G. Engineering the microflora to vaccinate the mucosa: Serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 2000, 100, 510–518.
- Norton, P.M.; Brown, H.W.; Wells, J.M.; Macpherson, A.M.; Wilson, P.W.; Le Page, R.W. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol. Med. Microbiol. 1996, 14, 167–177.
- Wells, J.M.; Wilson, P.W.; Norton, P.M.; Gasson, M.J.; Le Page, R.W. Lactococcus lactis: High-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 1993, 8, 1155–1162.
- Yang, X.Q.; Zhao, Y.G.; Chen, X.Q.; Jiang, B.; Sun, D.Y. The protective effect of recombinant Lactococcus lactis oral vaccine on a Clostridium difficile-infected animal model. BMC Gastroenterol. 2013, 13, 117.
- Jang, J.I.; Kim, J.S.; Eom, J.S.; Kim, H.G.; Kim, B.H.; Lim, S.; Bang, I.S.; Park, Y.K. Expression and delivery of tetanus toxin fragment C fused to the N-terminal domain of SipB enhances specific immune responses in mice. Microbiol. Immunol. 2012, 56, 595–604.
- Mazzantini, R.P.; Miyaji, E.N.; Dias, W.O.; Sakauchi, D.; Nascimento, A.L.T.O.; Raw, I.; Winter, N.; Gicquel, B.; Rappuoli, R.; Leite, L.C.C. Adjuvant activity of Mycobacterium bovis BCG expressing CRM197 on the immune response induced by BCG expressing tetanus toxin fragment C. Vaccine 2004, 22, 740–746.
- Romanos, M.A.; Makoff, A.J.; Fairweather, N.F.; Beesley, K.M.; Salter, D.E.; Rayment, F.B.; Payne, M.M.; Clare, J.J. Expression of tetanus toxin fragment C in yeast: Gene synthesis is required to eliminate fortuitous polyadenylation sites in AT-rich DNA. Nucleic Acids Res. 1991, 19, 1461–1467.
- Clare, J.J.; Rayment, F.B.; Ballantine, S.P.; Sreekrishna, K.; Romanos, M.A. High-level expression of tetanus toxin fragment C in Pichia pastoris strains containing multiple tandem integrations of the gene. Biotechnology 1991, 9, 455–460.
- Wang, N.; Wang, K.Y.; Xu, F.; Li, G.; Liu, D. The effect of N-glycosylation on the expression of the tetanus toxin fragment C in Pichia pastoris. Protein Expr. Purif. 2020, 166, 105503.
- Tregoning, J.S.; Nixon, P.; Kuroda, H.; Svab, Z.; Clare, S.; Bowe, F.; Fairweather, N.; Ytterberg, J.; van Wijk, K.J.; Dougan, G.; et al. Expression of tetanus toxin fragment C in tobacco chloroplasts. Nucleic Acids Res. 2003, 31, 1174–1179.
- Tregoning, J.S.; Maliga, P.; Dougan, G.; Nixon, P.J. New advances in the production of edible plant vaccines: Chloroplast expression of a tetanus vaccine antigen, TetC. Phytochemistry 2004, 65, 989–994.
- Yagi, Y.; Shiina, T. Recent advances in the study of chloroplast gene expression and its evolution. Front. Plant Sci. 2014, 5, 61.
- Ma, J.K.-C.; Drake, P.M.W.; Christou, P. Genetic modification: The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794–805.
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and high-level production of recombinant protein in plant chloroplasts using a temporary immersion bioreactor. Plant Biotechnol. J. 2011, 9, 575–584.
- Tregoning, J.S.; Clare, S.; Bowe, F.; Edwards, L.; Fairweather, N.F.; Qazi, O.; Nixon, P.J.; Maliga, P.; Dougan, G.; Hussell, T. Protection against tetanus toxin using a plant-based vaccine. Eur. J. Immunol. 2005, 35, 1320–1326.
- Charles, I.G.; Rodgers, B.C.; Makoff, A.J.; Chatfield, S.N.; Salter, D.E.; Fairweather, N.F. Synthesis of tetanus toxin fragment C in insect cells by use of a baculovirus expression system. Infect. Immun. 1991, 59, 1627–1632.
- Bayart, C.; Peronin, S.; Jean, E.; Paladino, J.; Talaga, P.; Borgne, M.L. The combined use of analytical tools for exploring tetanus toxin and tetanus toxoid structures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1054, 80–92.
- Lee, S.; Belitsky, B.R.; Brown, D.W.; Brinker, J.P.; Kerstein, K.O.; Herrmann, J.E.; Keusch, G.T.; Sonenshein, A.L.; Tzipori, S. Efficacy, heat stability and safety of intranasally administered Bacillus subtilis spore or vegetative cell vaccines expressing tetanus toxin fragment C. Vaccine 2010, 28, 6658–6665.
- Chai, P.; Pu, X.; Li, J.; Xia, X.; Ge, J.; Luo, A.; Su, H.; Zhang, W.; Ma, J. Expression and purification of tetanus toxin fragment C in Escherichia coli BL21(DE3). Protein Pept. Lett. 2020, 27, 1132–1140.
