1. General Remarks about the Mechanisms of Probiotics
The qualitative and quantitative composition of the gut microbiota has a major impact on the interaction with the host. The current strategy for treating dysbiosis is still largely based on the use of probiotics to restore microbial diversity and normalise the “disturbed” gut microbiota [
39]. Microbiota acts at different levels through complex mechanisms that are not fully understood [
40]. As a result, studies of the potential mechanistic actions of probiotics often encounter a number of limitations:
-
The use of oversimplified in vitro models that often fail to reproduce the results in vivo;
-
The use of “human” probiotics in animal models in vivo, (e.g., rodents), which do not take into account the functional significance of the species- and strain-specific administration of probiotics and their impact on the host’s immune response and its microbiota;
-
Probiotics’ action ultimately requires the involvement of the endogenous microbiota, which is host-specific and often diet-dependent and therefore hardly reproducible;
-
Most of the mechanisms of probiotics presented in research studies have suggested two main principles of probiotics’ action [
41];
-
Direct, contact-dependent principle (binding to different surface molecules);
-
Indirect principle via secretory molecules (production of bioactive peptides and metabolites).
2. Effect of Probiotics on Blood Parameters
2.1. In Vivo Human Studies
A number of human clinical trials demonstrated differential effects of probiotics on blood parameters in patients with various metabolic problems. These parameters reflect a direct or indirect relationship with glucose and its concentration in the blood (glycaemia) (Table 1).
Several studies have measured various blood parameters related to glycaemic status that may indicate possible control of blood glucose levels (glycaemic control). These can be divided into blood parameters, which are directly related to glycaemic status, such as fasting blood glucose, or blood parameters, which are indirectly related to glycaemic status, such as insulin, glycohaemoglobin (HbA1c) and homeostasis model assessment-estimated insulin resistance index (HOM-IR). However, some authors have included additional blood parameters, indirectly related to glycaemic status, in their studies, which are explained in more detail in
Table 1. Tonucci et al. [
50] have shown that glycaemic control is improved by taking probiotic fermented milk for 6 weeks. In one trial, administration of a probiotic preparation containing
Lactobacillus casei Shirota [
56] after 4 weeks maintained glycaemic control and preserved insulin levels in fasting state. A 6-month human clinical trial also reported beneficial effects of two probiotics (
Lactobacillus reuteri ADR-1 and ADR-3) on patients with type 2 diabetes. ADR-3 reduced some parameters of glycaemic control, while ADR-1 had a beneficial effect on lowering blood pressure [
51]. In another 12-week human clinical trial, probiotic did not alter HbA1c levels, fat levels or total serum bile acids [
54]. Furthermore, Shellekens et al. [
45] have demonstrated some anti-obesity effects of
Bifidobacterium longum APC1472 in humans, possibly due to an alternation in ghrelin signalling. Since the gut hormone ghrelin may be involved in glucose homeostasis via inhibition of insulin secretion [
57], administration of
Bifidobacterium longum APC1472 may be clinically significant in patients with pre-diabetes and type 2 diabetes mellitus. In addition, the authors speculated that the reduction in fasting cortisol levels due to probiotic administration affects the hypothalamic–pituitary–adrenal (HPA) axis, which regulates the pancreas and simultaneously influences glucagon and insulin secretion. All of these effects may contribute to glucose level reduction. A 12-week administration of
Lactobacillus plantarum OLL2712 in pre-diabetic patients has shown improvement in fasting plasma glucose levels, glycoalbumin levels and insulin resistance [
46]. In a trial in healthy adults, administration of the paraprobiotic
Lacticaseibacillus casei 01 resulted in a better reduction in blood glucose levels than the probiotic of the same name [
42].
The HOM-IR index is an important parameter in the context of cardiological and metabolic disorders, as it contributes to the early detection of insulin resistance and to the assessment of the risk of developing diabetes, cardiovascular pathologies and atherosclerosis [
58]. The effect of probiotics on blood parameters was also demonstrated in a clinical trial conducted in the Arab population [
47], in which a 6-month intake of a multi-strain probiotic supplement (Ecologic
® Barrier) showed a decrease in the HOM-IR index as well as a decrease in the level of endotoxin and inflammatory adipokines. The authors suggested the use of the probiotic supplement as a dietary supplement for patients with type 2 diabetes, but also emphasised the wide variation in results due to genetic diversity, dietary habits and environmental differences (geographical regions) between the populations studied [
47]. Kobyliak et al. [
52] also demonstrated the impact of a multi-strain probiotic (“Symbiter”) on reducing HOM-IR. Similar results were reported by Khalili et al. with
Lactobacillus casei 01 [
49] and
Lactobacillus casei [
48] as a supplement. In patients with metabolic syndrome, a two-month intake of probiotic yoghurt (
Lactobacillus acidophilus La5 and
Bifidobacterium lactis Bb12) led to a reduction in blood glucose levels and significant changes in insulin resistance (HOMA-IR) and sensitivity. The authors therefore believe that regular consumption of probiotic yoghurt has a positive effect on the treatment of metabolic syndrome [
44].
Interleukin-6 (IL-6), together with monocytochemotactic protein-1 (MCP-1), is a pro-inflammatory cytokine associated with the development of insulin resistance and hyperglycaemia [
52]. In addition, a pro-inflammatory interleukin 1 beta (IL-1β) cytokine may be involved in postprandial inflammation and thus in the regulation of glucose homeostasis and immune response [
59], while tumour necrosis factor alpha (TNF-α) seems to be mainly involved in insulin signalling and action [
60] and in the regulation of glucose and lipid metabolism [
61]. However, Kobylak et al. additionally showed that glycaemia-related parameters such as body weight, body mass index (BMI) and IL-6 remained unchanged or did not significantly decrease (HbA1c) after administration of multi-strain probiotics. However, the same compounded probiotics altered some pro-inflammatory factors such as TNF-α and IL-1β [
52]. Toshimitsu et al. [
46] have shown that both parameters as well as IL-6 were suppressed after 12 weeks of treatment with
Lactobacillus plantarum OLL2712 in a pilot trial with pre-diabetic patients.
HbA1c is an important parameter in the long-term monitoring of blood glucose levels (balance) and indicates the number of glucose molecules bound to haemoglobin in the red blood cells (erythrocytes) [
62]. The mechanism by which probiotics reduce the amount of this parameter is still unclear [
26]. Five clinical studies [
43,
50,
51,
52,
53] showed a statistically significant reduction in HbA1c levels after taking a probiotic preparation, while two other studies found no change in the amount of this parameter [
49,
63].
Fetuin-A is known as a glycoprotein, which is secreted by liver and adipose tissue. Its increase in the blood is indirectly related to patients with atherosclerosis, insulin resistance, diabetes mellitus and metabolic syndrome [
64], as it affects the activity of insulin receptor tyrosine kinase [
65]. Sirtuins (SIRTs) are very important regulators of energy homeostasis and metabolism due to their deacetylation activity in variety of organs [
66]. Fetuin-A and SIRTs were investigated in a randomised controlled trial in patients with type 2 diabetes after an 8-week intake of
Lactobacillus casei 01 and
Lactobacillus casei [
48,
49]. The results of blood analysis showed that fetuin-A levels were decreased, while SIRTs levels were increased.
Despite the proven beneficial effects of probiotic therapy in patients with type 2 diabetes, some studies failed to demonstrate changes in blood and metabolic parameters or the changes were not statistically significant, which is a crucial justification for a more objective approach and evaluation of the effectiveness of probiotic therapy. The diversity of results is mostly a consequence of the different methodological approaches in the studies: use of one or more probiotic strains, their amount and duration of administration and the number of patients. Moreover, it is already known that the activity of probiotics is species-specific [
67]. A comparison of the different results of the individual clinical probiotic studies can be carried out within the framework of meta-analytical studies. In this way, a more objective view of the potentially beneficial effect of probiotics on various blood and metabolic parameters in patients with type 2 diabetes can be obtained [
67,
68,
69,
70,
71,
72,
73,
74,
75,
76]. However, the above meta-analyses point to numerous limitations, even if they ultimately confirm the multiple beneficial effects of probiotics on blood and metabolic parameters: small groups of study participants [
67,
68,
69,
71,
74], lack of data on dietary habits and physical activity of study participants [
68,
70,
73], diversity within a study [
69,
71,
72,
73,
74], short duration of probiotic therapy, different therapeutic doses, use of different probiotic strains [
67,
68,
70,
72,
74], lack of evidence on possible side-effects of probiotic use [
69] and lack of testing of other metabolic factors affecting glycaemic control [
70,
71]. Last, but not least, Tao et al. [
76] proposed that more clinical data and research on the mechanisms of probiotic are needed.
2.2. In Vivo Animal Models Studies
Many studies in animal models demonstrated positive effects of various probiotics on diverse blood parameters. The range of measured parameters was similar to in vivo human studies showing potential effects of probiotics on improving glucose tolerance, insulin resistance and insulin sensitivity.
Oral intake of probiotic preparations of the strains
Lactobacillus casei and
Bifidobacterium bifidum (alone or both simultaneously) reduced the incidence of hyperglycaemia (increased blood glucose concentration) and dyslipidaemia (imbalance of blood lipid concentration) in rats with induced diabetes [
79]. Similar antidiabetic effects in rats were obtained by administration of probiotic strains
Lactobacillus plantarum TN627 [
80],
Bifidobacterium animalis 01 [
81],
Lactobacillus paracasei HII01 [
82], 9 strains of
Lactobacillus rhamnosus,
Bifidobacterium adolescentis and
Bifidobacterium bifidum [
77],
Lactobacillus rhamnosus BSL and R23 [
83], heat-inactivated
Streptococcus thermophilus [
84],
Lactiplantibacillus plantarum IMC 510 [
85] and
Lactobacillus fermentum RS-2 [
86]. In the latter case, in addition to reduced glucose levels in fasting state, a low oxidative stress was observed [
86].
Studies in mice and rats have shown, among other things, a positive effect of probiotic preparations on glucose balance [
78,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
112,
113,
118] and reduced insulin resistance [
78,
87,
89,
90,
91,
92,
94,
113]. Andersson et al. [
97], Sakai et al. [
98], Park et al. [
114], Naito et al. [
99], Wang et al. [
100,
101], Machado et al. [
102], Lee et al. [
103], Hsu et al. [
115], Kim et al. [
116] and Zeng et al. [
104] have shown that the probiotic addition of different species of the genus
Lactobacillus or a combination of probiotics [
100,
101,
116] improves glucose sensitivity (tolerance) [
97,
98,
99,
100,
101,
102,
103,
114,
115,
116] and reduces insulin resistance [
97,
98,
99,
100,
101,
114] by decreasing serum glucose levels in obese/diabetic mice and rat models. Endotoxaemia, along with inflammation, is a common factor in metabolic diseases (especially obesity, insulin resistance and diabetes). In a study conducted in mice, the addition of
Lactobacillus casei Shirota reduced insulin resistance and endotoxaemia. In this context, it has been demonstrated that the probiotic effect on glucose regulation is not necessarily directly related to the body weight and the amount of fat in the animals [
99].
Effects on glucose homeostasis and cholesterol metabolism have also been demonstrated by Ashrafian et al. [
105], Wang et al. [
106], Kim et al. [
116], Tarrah et al. [
107] and Sun et al. [
108]. In the latter study in mice,
Lactobacillus acidophilus SJLH001 had a statistically significant effect on the regulation of transcription genes, essential for glucose transport and cholesterol metabolism [
108]. Yan et al. [
110] have demonstrated a beneficial effect of
Lactobacillus acidophilus on type 2 diabetes in mice by controlling liver glucose levels, lipid metabolism and gut microbiota.
Furthermore, in a study on mice [
78,
91,
94,
113,
117] and rats [
84,
89], serum levels of certain inflammatory markers such as IL-6, TNF-α and IL-1β were decreased after a specific probiotic administration. In addition, heat-killed
Streptococcus thermophilus [
84],
Bifidobacterium animalis 01 [
81] and
Lactobacillus casei LC89 [
91],
Lactobacillus plantarum CCFM0236 [
78] increased the levels of IL-10, an important anti-inflammatory marker, when administered to rats and mice with type 2 diabetes, respectively.
A study in rats [
81] showed a statistically significant reduction in HbA1c levels after taking probiotic supplements. In addition to ageing and neurodegenerative diseases, impaired memory function in the brain may also be associated with impaired glucose metabolism, i.e., an increase in plasma HbA1c levels [
117]. Moreover, Bonfili et al. have demonstrated the beneficial effects of the probiotic formulation SLAB51 in a mouse model of Alzheimer’s disease. The probiotic formulation lowered serum HbA1c levels, showing a positive effect on glucose homeostasis [
111].
Osteocalcin is a protein hormone produced by osteoblasts. Primarily it inhibits bone formation, but as a hormone it plays an important role in the synthesis of testosterone in testis as well as synthesis of the muscle mass [
119]. However, it has been proven to have a positive effect on improving glucose tolerance and regulating glucose metabolism [
119,
120,
121]. The addition of
Lactobacillus casei Zhang in hyperinsulinaemic rats increased the amount of osteocalcin, which in turn influenced the improvement of glucose tolerance. The regulation of glucose tolerance is probably induced via the osteocalcin–adiponectin pathway [
109].
2.3. In Vitro Studies
The enzyme alpha-glucosidase (α-glucosidase) catalyses the digestive processes of carbohydrates and is responsible for increased blood glucose levels [
118]. In in vitro studies [
78,
94,
108,
118,
122], certain probiotics inhibited α-glucosidase activity and thereby decreasing the level of glucose ready for absorption and thereby glycaemic index of food.
3. Effect of Probiotics on Brain Function
The apparent communication between the intestinal microbiota and the central nervous system via the “gut-brain axis” has led researchers and/or clinicians to investigate the preventive and therapeutic use of probiotics in various neurological disorders [
123].
Recent studies have revealed an important role of glucose homeostasis in some brain pathological conditions, such as Alzheimer’s disease (AD) [
117]. Bonfili et al. have shown that the administration of probiotic SLAB51 increased glucose uptake in mice with Alzheimer’s disease due to the restoration of glucose receptor 3 (GLUT3) and glucose receptor 1 (GLUT1), the key glucose receptors in brain. The increased expression of both glucose receptors was in line with alleviated levels of phosphorylated 5′ adenosine monophosphate-activated protein kinase (AMPK) and protein kinase B (AKT), which are important metabolic regulators in most cell types. Moreover, they reported an increased expression of brain insulin-like growth factor receptor β (IGF-IRβ). IGF-IRβ is considered as a promising therapeutic target in AD, because of its important part in proteasome-mediated removal of oxidised proteins [
111].
4. Effect of Probiotics on Bile Acid Metabolism
Bile acids are highly effective surface-active digestive substances (surfactants) and natural emulsifiers whose main function is to support the digestion and absorption of fat-soluble lipids and vitamins. Their cholesterol production takes place exclusively in the hepatocytes. After they have fulfilled this function in the small intestine, they are reabsorbed into the bloodstream as part of the so-called “enterohepatic recirculation”. From here, their path leads back to the liver, where their activity is related to the various signalling pathways [
124].
Probiotics containing bile salt hydrolase (BSH) have been shown to interfere with the metabolism of bile acids and promote the deconjugation of bile salts via BSH, which is important in chronic metabolic diseases. In human studies, daily addition of
Lactobacillus reuteri NCIMB 30242 increased the amount of free bile acids that were no longer absorbed in the intestinal wall as a result of probiotic BSH deconjugation, but were excreted in the faeces. It was also speculated that deconjugation might be directly related to the increase in fibroblast growth factor 19 (FGF) [
125]. In fact, FGF plays an important role in the regulation of bile acid synthesis and its amount directly influences lipid and glucose metabolism [
126].
In a human clinical study,
Lactobacillus reuteri DSM 17938 can contribute to an increase in the level of deconjugated bile acids, which was also evidenced by the positive correlation with an increase in insulin sensitivity and thus an improvement in glucose metabolism. The authors hypothesised that the probiotic influences the biosynthesis of deconjugated bile acids by regulating genes for certain crucial enzymes involved in the biosynthesis [
54].
In a study in rats the probiotic
Lactobacillus casei Zhang [
109] increased gene expression of liver X-receptor-α (LXR), which has already been shown to increase glucose tolerance and cholesterol reduction via increased BSH levels and deconjugation of bile acids [
127]. In another study conducted in rats [
128], the same probiotic influenced the reduction of bacteria with hydrolase activity, resulting in increased bile acid secretion.
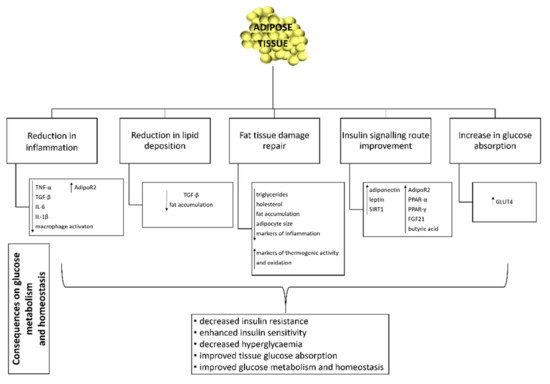
5. Effect of Probiotics on Adipose Tissue Function and Inflammation
For many years, adipose tissue was considered to be an inactive organ with a single function, the storage of lipids. However, a number of studies have shown that it is an active organ, which plays an essential metabolic and hormonal role in homeostasis of energy metabolism and glycaemic regulation [
16,
129] (
Figure 1). Excessive accumulation of lipids in the adipose tissue leads to its hypertrophy, cellular stress and local inflammation. The latter is chronically associated with obesity, insulin resistance, hyperglycaemia and type 2 diabetes [
130].
Figure 1. Effect of probiotics on adipose tissue. TNF-α: tumour necrosis factor alpha; TGF-β: transforming growth factor beta; IL-6: interleukin-6; IL-1β: interleukin 1 beta; AdipoR2; adiponectin receptor 2; TGF-β: transforming growth factor beta; SIRT1: sirtuin (silent mating type information regulation 2 homolog) 1; PPARα: peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor gamma; FGF21: fibroblast growth factor 21; GLUT4: glucose transporter 4; ↓: decrease; ↑: increase.
In studies on high-fat diet fed mice, some probiotic strains have demonstrated their protective role against chronic inflammation in adipose tissue, leading either to its prevention or its inhibition by reducing insulin resistance and hyperglycaemic state. Indeed, a high-fat diet has been shown to increase some of the pro-inflammatory factors such as fat TNF-α, IL-6, IL-1β.
Lactobacillus fermentum MTC5689 decreased the expression of pro-inflammatory indicator genes, TNF-α and IL-6 in visceral adipose tissue of mice [
131]. In the second study in mice,
Lactobacillus sakei OC67 reduced the incidence of hyperglycaemia and obesity by lowering inflammation [
132]. In both studies, as well as in three other studies [
98,
133,
134] reductions in glucose levels in fasting state and a decrease in IL-1β expression have been reported. The reduction of TNF-α expression in fat has also been shown after the addition of
Lactobacillus fermentum LM1016 [
133],
Lactobacillus crustorum MN047 and
Lactobacillus rhamnosus LS-8 [
134]. The later probiotic decreased the IL-6 expression in adipose tissue as well [
134]. The effect of
Lactobacillus rhamnosus GG also showed the improvement and reduction of glucose hypersensitivity and insulin resistance in obese mice. The addition of the probiotic was able to reduce the expression of genes for specific macrophage factors important for macrophage infiltration and activation, leading to a reduction in macrophage activation, inflammation and thus improved insulin sensitivity in adipose tissue [
114].
Deng et al. [
135] suggested that different genotypes of
Akkermansia municiphila exert specific effects in repairing damage to adipose tissue caused by a high-fat diet in mice. The study showed that different genotypes of probiotic can play different roles in the same disease state, particularly in combating endotoxaemia, inflammation and whitening of brown adipose tissue, glucose tolerance, hyperlipidaemia and hepatic steatosis.
Strains of the genera
Bifidobacterium [
136] and
Bacillus [
113] have been shown to influence the increased expression of proteins involved in the insulin signalling pathway in mice. Adiponectin, along with leptins, are involved in controlling feeding behaviour and increasing insulin sensitivity [
137]. Increased adiponectin mRNA levels in fat cells (adipocytes) [
136] and increased serum leptin levels [
113] have also been demonstrated in the above studies. Due to the increased insulin sensitivity, probiotics influenced the improvement of glucose absorption in tissues by elevating adiponectin and leptin concentrations, which led to a decrease in blood glucose levels [
138]. However, in a study in mice [
139], adiponectin blood levels as well as its mRNA levels in adipose tissue were decreased and restored by the administration of probiotic supernatants.
Peroxisome proliferator-activated receptor α (PPARα) is important for processes controlling energy expenditure, inflammation and glucose homeostasis [
140]. Due to increased levels of a hepatokine mediator of adiponectin expression in the regulation of glucose and lipid metabolism-fibroblast growth factor 21 (FGF21), increased PPARα mRNA levels and butyric acid levels, the authors suggested a possible link between probiotic mechanism-butyric acid-PPARα-FGF21-adiponectin-glucose and lipid metabolism [
139]. Indeed, Molina-Tijeras et al. [
141] linked the improvement in glucose and lipid metabolism to the upregulation of PPARα expression after administration of
Lactobacillus fermentum CECT5716.
The downregulation of
sirt1 (sirtuin-silent mating type information regulation 2 homolog 1) gene expression is an indicator of metabolic dysregulation in many organs, including adipose tissue. In a study on mice,
Lactobacillus plantarum increased adiponectin levels, which may have led to an upregulation of
sirt1 gene expression and thus to an improvement in lipid metabolic dysfunction [
142].
The role of the major adiponectin receptor gene, i.e., adiponectin receptor 2 (AdipoR2), has been linked to insulin resistance and type 2 diabetes, where it plays a crucial role in glucose and lipid metabolism, inflammation and oxidative stress [
143]. In a study on rats [
109], the probiotic
Lactobacillus casei Zhang also increased the expression of AdipoR2 in fat cells. The same probiotic has also increased the expression of the genes for the glucose receptor 4 (GLUT4) and core receptor-peroxisome proliferator-activated receptor gamma (PPAR-γ). Both parameters consequently had a positive effect on oral glucose tolerance. PPAR-γ is a known receptor that maintains the expression of key glucose regulatory and liporegulatory molecules, important for insulin signal transduction [
144], while GLUT4 is the major glucose transporter for the facilitated transfer of glucose from blood to skeletal fat tissue and muscle [
145]. A possible link between improved glycaemic levels, utilisation of glucose and increased expression of
GLUT4 genes following administration of
Lactobacillus fermentum CECT5716 has also been proposed [
141]. In addition to PPAR-γ, some probiotics have also been shown to affect increased expression of PPAR-α. in animal models [
105,
146] and in one in vitro study [
147]
A transforming growth factor beta (TGF-β) is known to be an important parameter in adipose tissue due to its effects on inflammatory mediators, energy homeostasis, fat expansion and collagen deposition [
148,
149]. A decrease in TGF-β mRNA levels after administration of
Akkermansia muciniphila as well as its extracellular vesicles (EVs) suggested a potential antidiabetic and anti-obesity effect of both the bacterium and its EVs [
105].
Lactobacillus plantarum Ln4 significantly reduced lipid deposition and induced glucose absorption in 3T3-L1 adipocytes in an in vitro study [
150].
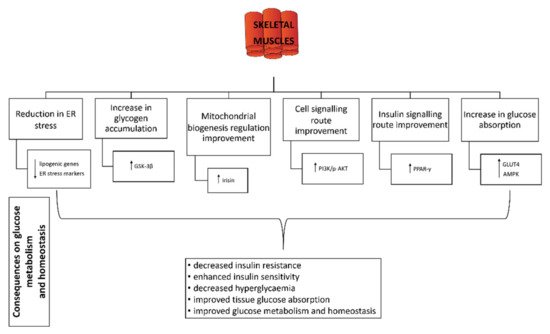
6. Effect of Probiotics on Skeletal Muscle
The endoplasmic reticulum (ER) plays a role as a sensor for changes in the homeostasis of a cell function. Stress therefore disrupts the normal function of the ER, which in turn reacts differently to protect the cell. It is known that lipid metabolism and ER stress are closely linked in skeletal muscle cells. In addition, ER stress in muscle cells is also a major cause of insulin resistance, which in turn affects glucose homeostasis [
151]. In a model study in mice, certain strains of
Lactobacillus species have been shown to influence the reduction of ER stressors in skeletal muscle, thereby reducing lipotoxicity. In fact, the probiotic effect showed a decrease in the expression of certain genes that can alter lipid metabolism [
114,
131].
Administration of
Lactobacillus casei or
Bifidobacterium bifidum strains or a combination of both significantly increased the amount of muscle glycogen in rats with induced diabetes, which had a direct effect on reducing hyperglycaemia [
79]. Similarly, Liu et al. showed an increase in protein levels of some glucose homeostasis-related molecules such as phosphoinositide 3-kinase (PI3K), phosphorylated protein kinase B (p-AKT) and glycogen synthase kinase 3 beta (GSK-3β). The latter is directly related to increased glycogen production [
139].
The protective effect of
Lactobacillus plantarum was demonstrated in a study conducted on mice [
142]. The expression of irisin, a specific adipomyokine produced by skeletal muscle and adipose tissue, was upregulated, which in turn increased the insulin sensitivity of skeletal muscle and improved hepatic glucose and lipid metabolism. Since irisin is a peroxisome proliferator-activated receptor-γ-coactivator (PGC)-1α-dependent molecule, the authors speculated on a possible concurrent probiotic mechanism for improving metabolism based on the regulation of mitochondrial biogenesis.
Kim et al. [
152] have also demonstrated the effect of a probiotic on increased expression of genes relevant to glycogen synthesis (glycogen synthesis-related gene pp-1) and increased expression of GLUT4 in skeletal muscle. In the same in vitro study, the authors showed improved insulin-dependent glucose uptake and increased expression of the GLUT4 and PPAR-γ genes.
The increase in GLUT4 expression due to the effect of the probiotic additive has been demonstrated in three animal studies: in mice [
153] and rats [
82,
146]. Toejing et al. [
82] linked the activation of GLUT4 translocation to the membrane in skeletal muscles to AMPK activation, which consequently affects the glucose uptake. The effects of probiotics on skeletal muscle in relation to glucose homeostasis and metabolism are shown in
Figure 2.
Figure 2. Effect of probiotics on skeletal muscles. GSK-3β: glycogen synthase kinase 3 beta; PI3K/AKT: phosphoinositide 3-kinase/phosphorylated protein kinase B signalling pathway; PPAR-γ: peroxisome proliferator-activated receptor gamma; GLUT4: glucose transporter 4; AMPK: 5′ adenosine monophosphate-activated protein kinase; ↓: decrease; ↑: increase.
7. Effect of Probiotics on Liver, Pancreas and Kidney
A pathological state of diabetes reduces the antioxidant potential of pancreatic and hepatic tissue and consequently increases the harmful effects of free radicals [
154]. Studies on animal models have shown that single probiotics reduce oxidative stress in the pancreas [
79,
104,
155], liver [
90,
93,
104,
156] and kidney [
93,
104], thus helping to increase the antioxidant activity and physiological function of the organ.
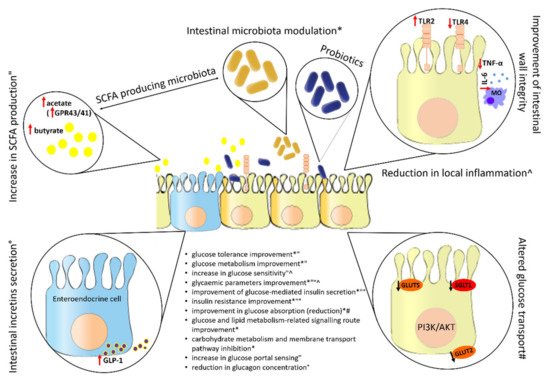
8. Effect of Probiotics on the Intestine
After ingestion, the intestine is the target organ of the probiotic and therefore of utmost importance for its various mechanisms of action. Figure 5 shows some common effects of probiotics in the intestine and the impact of their action on glucose metabolism and homeostasis.
Figure 5. Effect of probiotics on intestine. SCFA: short-chain fatty acids; PI3K/AKT: phosphoinositide 3-kinase/phosphorylated protein kinase B signalling pathway; GPR43/41: G protein-coupled receptor 43/41; GLP-1: glucagon-like polypeptide-1; TLR4: toll-like receptor 4; TLR2: toll-like receptor 2; TNF-α: tumour necrosis factor alpha; IL-6: interleukin-6; GLUT2: glucose transporter 2; GLUT5: glucose transporter 5; SGLT1: sodium-glucose cotransporter 1; ↓: decrease; ↑: increase; *, ", ^, °, #: each symbol stands for a different probiotic effect, which has a corresponding effect on glucose metabolism and homeostasis.
This entry is adapted from the peer-reviewed paper 10.3390/life12081187