Aptamers are DNA and RNA oligonucleotides that can adopt tridimensional structures that enable them to join specifically to any desired target. Aptamers are capable of binding to specific molecules including drugs, proteins, carbohydrates, cells, and viruses. Aptamers were first described in 1990, and since then several groups have used their binding properties to isolate a diversity of specific aptamers. Recently, aptamers have been studied for treatment and detection of many diseases including cancer. In Prostate Cancer, numerous works have reported their use in the development of new approaches in diagnostics and treatment strategies. Aptamers have been joined with drugs or other specific molecules such as silencing RNAs (aptamer–siRNA chimeras) to specifically reduce the expression of oncogenes in PCa cells. Even though these studies have shown good results in the early stages, more research is still needed to demonstrate the clinical value of aptamers in PCa.
- prostate cancer
- aptamers
- PCa diagnosis
- PCa treatment
1. Aptamers in Cancer
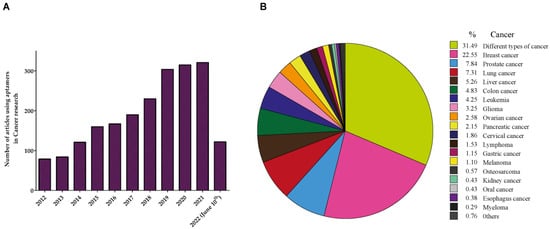
2. Aptamers in Prostate Cancer (PCa)
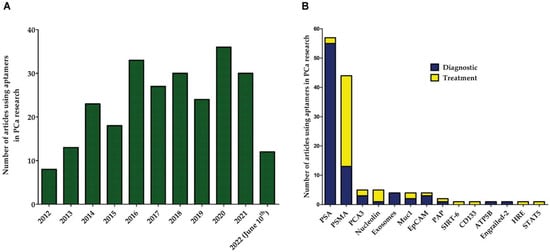
3. Aptamers against Prostate-Specific Membrane Antigen (PSMA)
3.1. PSMA Aptamers in PCa Diagnosis
3.2. PSMA Aptamers in PCa Therapy
3.3. Chimeras of PSMA
3.4. PSMA Aptamers as Vehicles in PCa
4. Other Aptamers in PCa
This entry is adapted from the peer-reviewed paper 10.3390/biom12081056
References
- Dongxi Xiang; Conglong Zheng; Shu-Feng Zhou; Shuxi Qiao; Phuong Tran; Chunwen Pu; Yong Li; Lingxue Kong; Abbas Z. Kouzani; Jia Lin; et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics 2014, 5, 1083-1097, 10.7150/thno.11711.
- Guizhi Zhu; Xiaoyuan Chen; Aptamer-based targeted therapy. Advanced Drug Delivery Reviews 2018, 134, 65-78, 10.1016/j.addr.2018.08.005.
- Weihong Tan; Hui Wang; Yan Chen; Xiaobing Zhang; Haizhen Zhu; Chaoyong Yang; Ronghua Yang; Chen Liu; Molecular aptamers for drug delivery. Trends in Biotechnology 2011, 29, 634-640, 10.1016/j.tibtech.2011.06.009.
- Fernando Pastor; Pedro Berraondo; Iñaki Etxeberria; Josh Frederick; Ugur Sahin; Eli Gilboa; Ignacio Melero; An RNA toolbox for cancer immunotherapy. Nature Reviews Drug Discovery 2018, 17, 751-767, 10.1038/nrd.2018.132.
- Sridharan Soundararajan; Li Wang; Vijayalakshmi Sridharan; Weiwei Chen; Nigel Courtenay-Luck; David Jones; Eleanor K. Spicer; Daniel J. Fernandes; Plasma Membrane Nucleolin Is a Receptor for the Anticancer Aptamer AS1411 in MV4-11 Leukemia Cells. Molecular Pharmacology 2009, 76, 984-991, 10.1124/mol.109.055947.
- Pooria Safarzadeh Kozani; Pouya Safarzadeh Kozani; Mohammad Tariq Malik; AS1411-functionalized delivery nanosystems for targeted cancer therapy. Exploration of Medicine 2021, 2, 146-166, 10.37349/emed.2021.00039.
- Caroline Madeleine Berger; Xavier Gaume; Philippe Bouvet; The roles of nucleolin subcellular localization in cancer. Biochimie 2015, 113, 78-85, 10.1016/j.biochi.2015.03.023.
- Sridharan Soundararajan; Weiwei Chen; Eleanor K. Spicer; Nigel Courtenay-Luck; Daniel J. Fernandes; The Nucleolin Targeting Aptamer AS1411 Destabilizes Bcl-2 Messenger RNA in Human Breast Cancer Cells. Cancer Research 2008, 68, 2358-2365, 10.1158/0008-5472.can-07-5723.
- Man Chen; Yuanyuan Yu; Feng Jiang; Junwei Zhou; Yongshu Li; Chao Liang; Lei Dang; Aiping Lu; Ge Zhang; Development of Cell-SELEX Technology and Its Application in Cancer Diagnosis and Therapy. International Journal of Molecular Sciences 2016, 17, 2079, 10.3390/ijms17122079.
- Julia Hoellenriegel; Dirk Zboralski; Christian Maasch; Nathalie Y. Rosin; William G. Wierda; Michael J. Keating; Anna Kruschinski; Jan A. Burger; The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032-1039, 10.1182/blood-2013-03-493924.
- Inga Nachreiner; Ahmad Fawzi Hussain; Ulrich Wullner; Nikolaus Machuy; Thomas F. Meyer; Rainer Fischer; Ivo Meinhold‑Heerlein; Stefan Barth; Mehmet Kemal Tur; Elimination of HER3‑expressing breast cancer cells using aptamer‑siRNA chimeras. Experimental and Therapeutic Medicine 2019, 18, 2401-2412, 10.3892/etm.2019.7753.
- Vaishali Bagalkot; Omid C. Farokhzad; Robert Langer; Sangyong Jon; An Aptamer–Doxorubicin Physical Conjugate as a Novel Targeted Drug-Delivery Platform. Angewandte Chemie International Edition 2006, 45, 8149-8152, 10.1002/anie.200602251.
- Nianxi Zhao; Sung-Nan Pei; Jianjun Qi; Zihua Zeng; Swaminathan P. Iyer; Pei Lin; Ching-Hsuan Tung; Youli Zu; Oligonucleotide aptamer-drug conjugates for targeted therapy of acute myeloid leukemia. Biomaterials 2015, 67, 42-51, 10.1016/j.biomaterials.2015.07.025.
- Hyosook Jeong; Soo Hyeon Lee; Yeonju Hwang; Hyundong Yoo; Heesun Jung; Sun Hwa Kim; Hyejung Mok; Multivalent Aptamer-RNA Conjugates for Simple and Efficient Delivery of Doxorubicin/siRNA into Multidrug-Resistant Cells. Macromolecular Bioscience 2016, 17, 1600343, 10.1002/mabi.201600343.
- Jing-Jing Zhang; Fang-Fang Cheng; Ting-Ting Zheng; Jun-Jie Zhu; Versatile aptasensor for electrochemical quantification of cell surface glycan and naked-eye tracking glycolytic inhibition in living cells. Biosensors and Bioelectronics 2017, 89, 937-945, 10.1016/j.bios.2016.09.087.
- Mitsunori Ushigome; Tsuneyuki Ubagai; Hirokazu Fukuda; Naoto Tsuchiya; Takashi Sugimura; Jun Takatsuka; Hitoshi Nakagama; Up-regulation of hnRNP A1 gene in sporadic human colorectal cancers. International Journal of Oncology 2005, 26, 635-640, 10.3892/ijo.26.3.635.
- Laura K. Zerbe; Irene Pino; Ruben Pio; Pippa Cosper; Lori D. Dwyer-Nield; Amy M. Meyer; J. David Port; Luis Montuenga; Alvin M. Malkinson; Relative amounts of antagonistic splicing factors, hnRNP A1 and ASF/SF2, change during neoplastic lung growth: Implications for pre-mRNA processing. Molecular Carcinogenesis 2004, 41, 187-196, 10.1002/mc.20053.
- Yan Yan-Sanders; George J Hammons; Beverly D Lyn-Cook; Increased expression of heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP) in pancreatic tissue from smokers and pancreatic tumor cells. Cancer Letters 2002, 183, 215-220, 10.1016/s0304-3835(02)00168-4.
- Jinqiang Zhang; Shaohua Li; Fang Liu; Lanping Zhou; Ningsheng Shao; Xiaohang Zhao; SELEX Aptamer Used as a Probe to Detect Circulating Tumor Cells in Peripheral Blood of Pancreatic Cancer Patients. PLOS ONE 2015, 10, e0121920-e0121920, 10.1371/journal.pone.0121920.
- Sung-Chi Tsai; Lien-Yu Hung; Gwo-Bin Lee; An integrated microfluidic system for the isolation and detection of ovarian circulating tumor cells using cell selection and enrichment methods. Biomicrofluidics 2017, 11, 034122, 10.1063/1.4991476.
- Rebecca L. Siegel; Kimberly D. Miller; Ahmedin Jemal; Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 2016, 66, 7-30, 10.3322/caac.21332.
- Michael E Stokes; Jack Ishak; Irina Proskorovsky; Libby K Black; Yijian Huang; Lifetime economic burden of prostate cancer. BMC Health Services Research 2011, 11, 349-349, 10.1186/1472-6963-11-349.
- W. J. Catalona; Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA 1995, 274, 1214-1220, 10.1001/jama.274.15.1214.
- Per-Anders Abrahamsson; Hans Lilja; Joseph E. Oesterling; MOLECULAR FORMS OF SERUM PROSTATE-SPECIFIC ANTIGEN: The Clinical Value of Percent Free Prostate-Specific Antigen. Urologic Clinics of North America 1997, 24, 353-365, 10.1016/s0094-0143(05)70382-7.
- Eric H Kim; Gerald L Andriole; Prostate-specific antigen-based screening: controversy and guidelines. BMC Medicine 2015, 13, 61-61, 10.1186/s12916-015-0296-5.
- Alan W. Partin; H. Ballentine Carter; Daniel W. Chan; Jonathan I. Epstein; Joseph E. Oesterling; Robert C. Rock; Jed P. Weber; Patrick C. Walsh; Prostate Specific Antigen in the Staging of Localized Prostate Cancer: Influence of Tumor Differentiation, Tumor Volume and Benign Hyperplasia. Journal of Urology 1990, 143, 747-752, 10.1016/s0022-5347(17)40079-6.
- H. B. Carter; Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA 1992, 267, 2215-2220, 10.1001/jama.267.16.2215.
- Joseph A. Smith; Prevalence of prostate cancer among men with a prostate-specific antigen level ⩽4.0 ng per milliliter: Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia, MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA Jr., Division of Urology, Department of Surgery, University of Texas Health Science Center at San Antonio, San Antonio, TX. N Engl J Med 2004;350:2239–46. Urologic Oncology: Seminars and Original Investigations 2004, 22, 493-493, 10.1016/j.urolonc.2004.08.008.
- S.A. Tomlins; D.R. Rhodes; S. Perner; S.M. Dhanasekaran; R. Mehra; X.-W. Sun; S. Varambally; X. Cao; J. Tchinda; R. Kuefer; et al. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Journal of Urology 2006, 175, 1707-1707, 10.1016/s0022-5347(06)00096-6.
- Grégoire Robert; Sander Jannink; Frank Smit; Tilly Aalders; Daphne Hessels; Ruben Cremers; Peter F. Mulders; Jack A. Schalken; Rational basis for the combination of PCA3 and TMPRSS2:ERG gene fusion for prostate cancer diagnosis. The Prostate 2012, 73, 113-120, 10.1002/pros.22546.
- Indu Kohaar; Gyorgy Petrovics; Shiv Srivastava; A Rich Array of Prostate Cancer Molecular Biomarkers: Opportunities and Challenges. International Journal of Molecular Sciences 2019, 20, 1813, 10.3390/ijms20081813.
- Philip A. Watson; Vivek K. Arora; Charles L. Sawyers; Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nature Cancer 2015, 15, 701-711, 10.1038/nrc4016.
- Jeffrey S Ross; Christine E Sheehan; Hugh A G Fisher; Ronald P Kaufman; Prabhjot Kaur; Karen Gray; Iain Webb; Gary S Gray; Rebecca Mosher; Bhaskar V S Kallakury; et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer.. Clinical Cancer Research 2003, 9, 6357–6362, .
- Denise S. O’Keefe; Sai L. Su; Dean J. Bacich; Yutaka Horiguchi; Ying Luo; C.Thomas Powell; Dorothea Zandvliet; Pamela Russell; Peter Molloy; Norma J. Nowak; et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression 1998, 1443, 113-127, 10.1016/s0167-4781(98)00200-0.
- Sam S Chang; Victor E Reuter; W.D.W Heston; Paul B Gaudin; Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology 2001, 57, 1179-1183, 10.1016/s0090-4295(01)00983-9.
- M L Beckett; Lisa Cazares; Antonia Vlahou; P F Schellhammer; G L Wright; Prostate-specific membrane antigen levels in sera from healthy men and patients with benign prostate hyperplasia or prostate cancer.. Clinical Cancer Research 1999, 5, 4034–4040, .
- Michael L. Salgaller; Patricia A. Lodge; Joanne G. McLean; Ben A. Tjoa; Douglas J. Loftus; Haakon Ragde; Gerald M. Kenny; Mary Rogers; Alton L. Boynton; Gerald P. Murphy; et al. Report of immune monitoring of prostate cancer patients undergoing T-cell therapy using dendritic cells pulsed with HLA-A2-specific peptides from prostate-specific membrane antigen (PSMA). The Prostate 1998, 35, 144-151, 10.1002/(sici)1097-0045(19980501)35:2<144::aid-pros8>3.0.co;2-j.
- Vaishali Bagalkot; Liangfang Zhang; Etgar Levy-Nissenbaum; Sangyong Jon; Philip W. Kantoff; And Robert Langer; Omid C. Farokhzad; Quantum Dot−Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Letters 2007, 7, 3065-3070, 10.1021/nl071546n.
- Kyoungin Min; Kyung-Mi Song; Minseon Cho; Yang-Sook Chun; Yoon-Bo Shim; Ja Kang Ku; Changill Ban; Simultaneous electrochemical detection of both PSMA (+) and PSMA (−) prostate cancer cells using an RNA/peptide dual-aptamer probe. Chemical Communications 2010, 46, 5566-5568, 10.1039/c002524k.
- Xiaozhou Fan; Yanli Guo; Luofu Wang; Xingyu Xiong; Lianhua Zhu; Kejing Fang; Diagnosis of prostate cancer using anti-PSMA aptamer A10-3.2-oriented lipid nanobubbles. International Journal of Nanomedicine 2016, ume 11, 3939-3950, 10.2147/ijn.s112951.
- Supriya S. Pai; Andrew D. Ellington; Using RNA Aptamers and the Proximity Ligation Assay for the Detection of Cell Surface Antigens. null 2007, 504, 385-398, 10.1007/978-1-60327-569-9_21.
- Leila Farzin; Mojtaba Shamsipur; Recent advances in design of electrochemical affinity biosensors for low level detection of cancer protein biomarkers using nanomaterial-assisted signal enhancement strategies. Journal of Pharmaceutical and Biomedical Analysis 2017, 147, 185-210, 10.1016/j.jpba.2017.07.042.
- Annika Spruessel; Garnet Steimann; Mira Jung; Sung A. Lee; Theresa Carr; Anne-Kristin Fentz; Joerg Spangenberg; Carsten Zornig; Hartmut H. Juhl; Kerstin A. David; et al. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. BioTechniques 2004, 36, 1030-1037, 10.2144/04366rr04.
- Matias Knuuttila; Emrah Yatkin; Jenny Kallio; Saija Savolainen; Teemu D. Laajala; Tero Aittokallio; Riikka Oksala; Merja Häkkinen; Pekka Keski-Rahkonen; Seppo Auriola; et al. Castration Induces Up-Regulation of Intratumoral Androgen Biosynthesis and Androgen Receptor Expression in an Orthotopic VCaP Human Prostate Cancer Xenograft Model. The American Journal of Pathology 2014, 184, 2163-2173, 10.1016/j.ajpath.2014.04.010.
- Nicolai Mader; Daniel Groener; Nikolaos Tselis; Séverine Banek; James Nagarajah; Frank Grünwald; Amir Sabet; Outcome of 177Lu-PSMA-617 Radioligand Therapy in Chemo-Refractory Patients with Metastatic Castration-Resistant Early-Onset Prostate Cancer. Cancers 2021, 13, 4193, 10.3390/cancers13164193.
- Shanta Dhar; Nagesh Kolishetti; Stephen J. Lippard; Omid C. Farokhzad; Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proceedings of the National Academy of Sciences 2011, 108, 1850-1855, 10.1073/pnas.1011379108.
- Omid C. Farokhzad; Jianjun Cheng; Benjamin A. Teply; Ines Sherifi; Sangyong Jon; Philip W. Kantoff; Jerome P. Richie; Robert Langer; Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proceedings of the National Academy of Sciences 2006, 103, 6315-6320, 10.1073/pnas.0601755103.
- Zhongjian Chen; Zongguang Tai; Fenfen Gu; Chuling Hu; Quangang Zhu; Shen Gao; Aptamer-mediated delivery of docetaxel to prostate cancer through polymeric nanoparticles for enhancement of antitumor efficacy. European Journal of Pharmaceutics and Biopharmaceutics 2016, 107, 130-141, 10.1016/j.ejpb.2016.07.007.
- Wenjin Xu; Imtiaz A. Siddiqui; Minakshi Nihal; Srikanth Pilla; Kimberly Rosenthal; Hasan Mukhtar; Shaoqin Gong; Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials 2013, 34, 5244-5253, 10.1016/j.biomaterials.2013.03.006.
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S.; Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular alpha-particle therapy of cancer.. J. Nucl. Med. 2014, 55, 107-114, https://doi.org/10.2967/jnumed.113.125476.
- Dongkyu Kim; Yong Yeon Jeong; Sangyong Jon; A Drug-Loaded Aptamer−Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689-3696, 10.1021/nn901877h.
- Xiaohua Ni; Yonggang Zhang; Kenji Zennami; Mark Castanares; Amarnath Mukherjee; Raju R. Raval; Haoming Zhou; Theodore L. DeWeese; Shawn E. Lupold; Systemic Administration and Targeted Radiosensitization via Chemically Synthetic Aptamer–siRNA Chimeras in Human Tumor Xenografts. Molecular Cancer Therapeutics 2015, 14, 2797-2804, 10.1158/1535-7163.mct-15-0291-t.
- Pei Jing; Shousong Cao; Shuangli Xiao; Xiaoqin Zhang; Siyun Ke; Famin Ke; Xin Yu; Li Wang; Shurong Wang; Yuling Luo; et al. Enhanced growth inhibition of prostate cancer in vitro and in vivo by a recombinant adenovirus-mediated dual-aptamer modified drug delivery system. Cancer Letters 2016, 383, 230-242, 10.1016/j.canlet.2016.10.003.
- Ulrich Wullner; Inga Neef; Andreas Eller; Michael Kleines; Mehmet Kemal Tur; Stefan Barth; Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2.. Current Cancer Drug Targets 2008, 8, 554-565, 10.2174/156800908786241078.
- Justin P Dassie; Xiu-Ying Liu; Gregory S Thomas; Ryan M Whitaker; Kristina W Thiel; Katie R Stockdale; David Meyerholz; Anton P McCaffrey; James O McNamara II; Paloma H Giangrande; et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nature Biotechnology 2009, 27, 839-846, 10.1038/nbt.1560.
- Daniel W Binzel; Yi Shu; Hui Li; Meiyan Sun; Qunshu Zhang; Dan Shu; Bin Guo; Peixuan Guo; Specific Delivery of MiRNA for High Efficient Inhibition of Prostate Cancer by RNA Nanotechnology. Molecular Therapy 2016, 24, 1267-1277, 10.1038/mt.2016.85.
- 119. Jie, F.; Xiao-Lin, W.; Yu-Jie, L.; Jing, Z.; Fang, W.; Shen-Si, X.; Xin, G.; Qing-Qing, W.; Hai-Feng, S.; In vivo analysis of treatment of prostate cancer with prostate surface membrane antigen aptamer-cationic liposome-double siRNA complex.. Chin. J. Biol. 2016, 29, 151-156, .
- James O McNamara Ii; Eran Andrechek; Yong Wang; Kristi D Viles; Rachel E Rempel; Eli Gilboa; Bruce A Sullenger; Paloma H Giangrande; Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nature Biotechnology 2006, 24, 1005-1015, 10.1038/nbt1223.
- Shawn E Lupold; Brian J Hicke; Yun Lin; Donald S Coffey; Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen.. Cancer Research 2002, 62, 4029-4033, .
- Romain Mathieu; Ilaria Lucca; Mihai D. Vartolomei; Aurélie Mbeutcha; Tobias Klatte; Christian Seitz; Pierre I. Karakiewicz; Harun Fajkovic; Maxine Sun; Yair Lotan; et al. Role of survivin expression in predicting biochemical recurrence after radical prostatectomy: a multi-institutional study. BJU International 2016, 119, 234-238, 10.1111/bju.13472.
- John C. Leach; Andrew Wang; Kaiming Ye; Sha Jin; A RNA-DNA Hybrid Aptamer for Nanoparticle-Based Prostate Tumor Targeted Drug Delivery. International Journal of Molecular Sciences 2016, 17, 380, 10.3390/ijms17030380.
- Benyi Li; Changlin Li; Suppression of Prostate Cancer Metastasis by DPYSL3-Targeted saRNA. null 2017, 983, 207-216, 10.1007/978-981-10-4310-9_15.
- Xiaohua Ni; Yonggang Zhang; Kenji Zennami; Mark Castanares; Amarnath Mukherjee; Raju R. Raval; Haoming Zhou; Theodore L. DeWeese; Shawn E. Lupold; Systemic Administration and Targeted Radiosensitization via Chemically Synthetic Aptamer–siRNA Chimeras in Human Tumor Xenografts. Molecular Cancer Therapeutics 2015, 14, 2797-2804, 10.1158/1535-7163.mct-15-0291-t.
- 125. Diao, Y.; Liu, J.; Ma, Y.; Su, M.; Zhang, H.; Hao, X.; A specific aptamer-cell penetrating peptides complex delivered siRNA efficiently and suppressed prostate tumor growth in vivo. . Cancer Biol. Ther. 2016, 17, 498–506, https://doi.org/10.1080/15384047.2016.1156266.
- Yuying Jiao; Peng Xu; Sha Luan; Xinyu Wang; Yue Gao; Changjiu Zhao; Peng Fu; Molecular imaging and treatment of PSMA-positive prostate cancer with 99mTc radiolabeled aptamer-siRNA chimeras. Nuclear Medicine and Biology 2021, 104-105, 28-37, 10.1016/j.nucmedbio.2021.11.003.
- A.S. Kibel; Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Yearbook of Urology 2008, 2009, 157-158, 10.1016/s0084-4071(09)79258-9.
- 128. Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; Lavan, D.A.; Langer, R.; Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672, https://doi.org/10.1158/0008-5472.CAN-04-2550.
- Santosh Kumar Singh; Jennifer B. Gordetsky; Sejong Bae; Edward P. Acosta; Jr. James W. Lillard; Rajesh Singh; Selective Targeting of the Hedgehog Signaling Pathway by PBM Nanoparticles in Docetaxel-Resistant Prostate Cancer. Cells 2020, 9, 1976, 10.3390/cells9091976.
- Si Eun Baek; Kwang Hyun Lee; Yong Serk Park; Deok-Kun Oh; Sangtaek Oh; Keun-Sik Kim; Dong-Eun Kim; RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. Journal of Controlled Release 2014, 196, 234-242, 10.1016/j.jconrel.2014.10.018.
- A.S. Kibel; Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Yearbook of Urology 2008, 2009, 157-158, 10.1016/s0084-4071(09)79258-9.
- Meng Wu; Ying Wang; Yiru Wang; Mingbo Zhang; Yukun Luo; Jie Tang; Zhigang Wang; Dong Wang; Lan Hao; Zhibiao Wang; et al. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. International Journal of Nanomedicine 2017, ume 12, 5313-5330, 10.2147/ijn.s136032.
- Minlan Duan; Yuqian Long; Cai Yang; Xiaoqiu Wu; Yang Sun; Jianglin Li; Xiaoxiao Hu; Wei Lin; Dongmei Han; Yifan Zhao; et al. Selection and characterization of DNA aptamer for metastatic prostate cancer recognition and tissue imaging. Oncotarget 2016, 7, 36436-36446, 10.18632/oncotarget.9262.
- Yan Hu; Jinhong Duan; Qimin Zhan; Fengdan Wang; Xin Lu; Xian-Da Yang; Novel MUC1 Aptamer Selectively Delivers Cytotoxic Agent to Cancer Cells In Vitro. PLOS ONE 2012, 7, e31970, 10.1371/journal.pone.0031970.
- Zohreh Noaparast; Seyed Jalal Hosseinimehr; Majid Piramoon; Seyed Mohammad Abedi; Tumor targeting with a99mTc-labeled AS1411 aptamer in prostate tumor cells. Journal of Drug Targeting 2015, 23, 497-505, 10.3109/1061186x.2015.1009075.
- Esther Campos-Fernández; Letícia S. Barcelos; Aline G. Souza; Luiz R. Goulart; Vivian Alonso-Goulart; Post-SELEX Optimization and Characterization of a Prostate Cancer Cell-Specific Aptamer for Diagnosis. ACS Omega 2020, 5, 3533-3541, 10.1021/acsomega.9b03855.
- Shokoufeh Hassani; Armin Salek Maghsoudi; Milad Rezaei Akmal; Soheila Rahmani Rahmani; Pouria Sarihi; Mohammad Reza Ganjali; Parviz Norouzi; Mohammad Abdollahi; A Sensitive Aptamer-Based Biosensor for Electrochemical Quantification of PSA as a Specific Diagnostic Marker of Prostate Cancer. Journal of Pharmacy & Pharmaceutical Sciences 2020, 23, 243-258, 10.18433/jpps31171.
