1. Pharmacokinetics of Ole
1.1. Dose of Ole
Ole or olive leaf extract is available in a variety of formats as a dry extract [
8]. Some studies suggest that 7.5 g of Ole for a 70 kg human may have an anti-tumor effect by decreasing mitosis and by increasing apoptosis [
9,
10], but this high dose may be impossible to achieve because the commercially available supplement of the olive leave extract contains approximately 20 mg−50 mg of Ole [
11,
12,
13]. Ole at high levels, such as 50 mg/kg, is non-toxic [
14].
1.2. Metabolism of Ole
Ole is absorbed in the small intestine and colon of animals and humans [
15], undergoes high first-pass metabolism, forms sulfate and glucuronide conjugations [
16], is degraded to HT in the large intestine, binds circulating human lipoproteins, and is excreted in urine as glucuronide conjugates [
15]. The free form of Ole cannot be detected since it is quickly transformed into HT [
16]. Because ole is resistant to stomach acidity, it does not undergo hydrolysis in the stomach [
17].
It is poorly absorbed in the isolated perfused rat gut, according to Ole’s in situ test, whereas HT absorption is greater [
18]. It is worth noting that, according to one study, Ole is not the primary source of HT [
13]. In an experiment on freely moving rats, Ole showed the slowest absorption profile compared with HT, with just a little amount of Ole detected in plasma and urine following an oral dose (300 mg/kg; 555.02 µmol/kg) [
18]. Ole was found in the plasma 5 min after receiving IV therapy (10 mg/kg; 18.50 µmol/kg) and was not found in urine; however, it was found in bile [
18]. Ole metabolism is mediated by a number of metabolic processes, including de-glycosylation, hydrolysis, oxygenation, and methylation, according to that study, which explains why Ole was detected in bile [
19].
2. Role of Ole in Cancer
2.1. Anti-Proliferative Effects of Ole
Researchers have discovered that, following Ole treatment at various IC50 doses (0.5× IC50, IC50, and 2× IC50), the proliferation of in vitro MCF-7 breast cancer cells diminishes in a time-dependent way [
25]. Ole’s anti-proliferative action has been established in numerous research using MCF-7 cell lines (
Table 1). Researchers have noted that the benefits of Ole may be preventative rather than therapeutic [
26]. In vivo studies on mice that were subcutaneously injected with MCF-7 and given 125 mg/kg of Ole in their food revealed that it suppresses peri pulmonary and parenchymal lung metastases [
27].
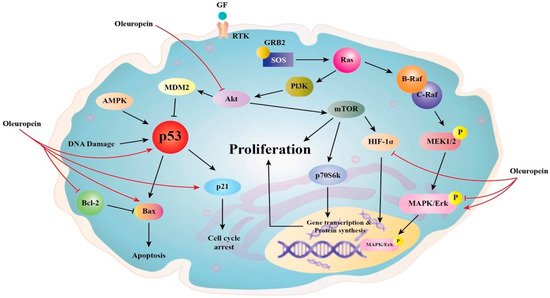
Ole (100 µM) was found to suppress the nuclear factor-light-chain-enhancer of activated B (NF-kB) and its downstream targets cyclin D1 and cyclooxygenase-2 (COX2) in the MDA-MB-231 breast cancer cell line. This impact is thought to be connected to the fact that Ole reduces the expression of serine/threonine kinase (Akt) and IB [
28], which are components of the NF-kB activation cascade (
Figure 2) [
29]. In vitro, Ole induced anti-proliferative effects on both TCAM-2 and SEM-1 cells by inhibiting the NF-κB pathway [
30]. Ole was also reported to not affect IB in HT 29 colon cancer at concentrations of 400 and 800 µM [
31]. The standard mechanism of NF-kB activation necessitates the phosphorylation of its inhibitor proteins, such as IB, but the non-canonical pathway does not [
32].
Figure 2. Proposed anti-proliferative mechanisms of Ole.
The COX2 pathway is linked to colon cancer because it promotes proliferation and angiogenesis [
33]. This is due to increased prostaglandin production. Ole was demonstrated to downregulate COX2. The suppression of the transcription factor cAMP response element-binding protein (CREB) is linked to this effect [
16]. The downregulation of COX2 in colon cancer could be linked to the downregulation of the wnt/-catenin pathway by Ole [
34]. Many malignancies, including gastric [
35], colon [
34], and endometrial [
36], were linked to hyperactivation of the wnt/-catenin pathway. Because of the capacity of the wnt/-catenin pathway to activate COX2, studies on the use of non-steroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and treatment have been conducted [
37].
B-cell lymphoma 2 (Bcl2); NF-kB; the wnt/-catenin pathway [
38]; and peroxisome proliferator-activated receptor (PPAR), which plays a key role in adipogenesis and is linked to obesity, insulin-dependent treatment [
39], and osteoporosis [
38], were discovered to modulate many gene expressions that promote adipogenesis but are also linked to cancer.
Because of its high expression in prostate, ovarian, cervical, follicular thyroid, esophageal, and testicular cancers, new studies looked at the role of PPAR in cancer [
40]. According to one study, PPAR agonists may increase the risk of colorectal cancer [
31]. High levels of PPAR expression have been associated with cell proliferation and tumor development inhibition [
40], but it is yet unclear whether this is beneficial. It was discovered that HT, not Ole, is responsible for upregulating PPAR gene expression in HT-29 colon cancer cells in vitro [
31].
Because several of these pathways are linked to the epithelial–mesenchymal transition [
41], evidence of Ole’s ability in preventing osteoporosis has led to the hypothesis that Ole can also prevent bone cancer proliferation [
6]. Ole can influence numerous pathways, including wnt/-catenin [
41] and MMP and their inhibitors, as well as upstream activators of NF-kB [
42] such as mitogen-activated protein kinases (p38 MAPK), extracellular signal-regulated kinase (ERK), and AKT [
43]. Therefore, it is possible that Ole can prevent bone tumor formation by altering the tumor microenvironment [
41]. The properties of the tumor microenvironment, or premetastatic niche, are significant aspects of cancer [
44]. Many of those influenced by Ole, including VEGF, MMP, and NF-kB, are involved in this niche.
Ole (370 µM) possesses an anti-metastatic effect in MDA-MB-231 cells, which is thought to be attributable to the increased tissue suppression of metalloproteinase activity, which improves apoptosis and inhibits the activity of matrix metalloproteinase, (MMP) (promotes tumor cell metastasis) [
5]. In glioma cells, the upregulation of MMP, which promotes invasion, was also observed. Ole (200/400 µM) considerably reduces the expressions of MMP-2 and MMP-9 in U251 and A172 hepatocarcinoma cells [
45], while Ole (400 µM) dramatically reduces MMP-7 in HepG2 hepatocarcinoma cells [
46]. MMP-2 plays a variety of roles in cancer angiogenesis, invasion, and tolerance [
47]. MMP-7 and MMP-9, on their own or in combination, can induce tumor angiogenesis [
48]. The mechanism by which MMP-9 and MMP-7 exert both angiogenic and pro-angiogenic effects is currently unknown, and more research is needed [
48]. The activation of the NF-kB pathway has been shown to enhance MMP-9 production [
27], and because Ole affects multiple components of the NF-kB pathway, the effects on MMP might be attributable to this [
28,
34].
In thyroid cancer cell lines, TPC-1 and BCPAP were used to confirm the antiproliferative activity of Ole and peracetylated Ole compared with non-tumor TAD-2 cell lines [
49]. It was demonstrated that the antiproliferative activity was seen at concentrations below 100 M. The reduced phosphorylation of ERK and Akt [
49], which are critical in cancer dissemination and invasion [
50,
51], was linked to this activity. Ole (100 and 500 µM) also inhibits LNCaP and DU145 prostate cell proliferation by causing necrosis [
52].
AMPK (5′ adenosine monophosphate-activated protein kinase), CK1 or p21 are Cyclin-dependent kinase inhibitors, GF (growth factor), GRB2 (growth factor receptor-bound protein 2), MEK (mitogen-activated protein kinase),
MDM2 (murine double minute 2), RAF (rapidly accelerated fibrosarcoma), RTK (receptor tyrosine kinase), p53 (tumor protein p53), and p70S6K (70-kDa ribosomal protein S6 kinase). The figure highlights Oleuropein’s main signaling effects in reducing cancer cell proliferation and survival as well as its influence on these molecules as a chemotherapeutic drug that induces apoptosis by inhibiting the AKT signaling cascade and p53. Oleuropein raises the expression of the proapoptotic proteins p53 and Bax while decreasing the expression of the antiapoptotic proteins Bcl-2 and HIF-1. It also targets particular pathways that inhibit AKT [
5].
Cannabinoid receptors (CB) are considered new anti-cancer targets because of their relevance to cancer progression and proliferation. In particular, CB1 is considered a tumor suppressor, and activation of this receptor initiates many cascades that prevent cancer onset, progression, and proliferation due to diverse mechanisms in multiple cancer cells, such as GI, lung, breast, and brain, prostate, pancreas, and thyroid [
53]. The proliferative effect of lowering CB1 levels in cancer is inhibited by the upregulation of this receptor through Ole (50 µM) administration in human colon Caco-2 cells [
54]. Ole (50 µM) also prevents the onset of colon cancer progression in vitro and in vivo through upregulation of the gene coding for CB1 [
16]. In SH-SY5Y neuroblastoma cells, Ole (350 µM) significantly inhibits cell migration in vitro [
55], and the mechanism behind this is still unknown.
2.2. Anti-Angiogenic and Apoptotic of Ole
Ole’s anti-angiogenic effect in in vivo breast cancer cell lines may be mediated by lowering the vascular endothelial growth factor (VEGF) at doses of 225 mg/kg/day for 3 weeks, administered in distilled water via gastric lavage [
9]. This conclusion is supported by the fact that Ole (150 and 225 mg/kg/day), in vivo, decreases breast tumor volume, and this is related to the increase in endostatin expression [
9].
In vivo and in vitro studies indicate that endostatin competes with VEGF to bind its receptor, which further prevents its phosphorylation and downstream pathway activation [
9,
56]. In the in vitro MDA-MB-321 breast cancer cell line, Ole induced cysteine proteases with aspartate specificity (caspase)-3 cleavage at a concentration of 200 µM, which resulted in the induction of cancer cell apoptosis [
14]. The same was also observed in the in vitro NSCLC H1299 lung cancer cell line in which Ole, at the same concentration, caused a significant increase in cytochrome c and thus caspase-3 [
57]. Ole’s effects on PARP cleavage might be modulated in part by caspase-3 cleavage at 100 µM concentration [
28]. A study conducted to elucidate the effects of Ole on gene expression of breast cancer cells showed that in MIDA-MB-468 in vitro cells, Ole (250 µM in water) increased the expression of many caspases including caspase 1 and 14, which have a role in initiating apoptosis [
58], while in MDA-MB-231 in vitro cells, Ole (500 µM in water) caused an increase in caspase 4 expression, which also promotes apoptosis [
58].
Bcl-2-associated X protein (Bax) and Bcl2 have been demonstrated to promote apoptosis in U251 and A172 glioma cells by increasing caspase-3 and 9 expressions [
45]. Bax and Bcl2 are proteins that regulate the apoptotic pathway in mitochondria through caspases. Bax is pro-apoptotic (promotes death), whereas Bcl2 is anti-apoptotic (prevents apoptosis and promotes survival).
Ole (200 µM) raised the ratio of Bax/Bcl2, favoring the apoptotic pathway in MIA PaCa-2 pancreatic cancer cells better than HT (100 µM), although this is not found in healthy cell lines [
59]. Similar effects were observed in NSCLC H1299 lung cancer cells in vitro at 200 µM [
57], breast neuroblastoma in vitro at 350 µM [
55], and Hela cervical cancer cells in vitro at 200 µM [
24]. Evidence suggests that Ole (200 µM) may affect the proapoptotic gene tumor protein P53 (p53) as well as the Bax/bcl2 ratio, which favors apoptosis. P53 and Bax are both proapoptotic, according to studies on MCF-7 in in vitro breast cancer cells [
25]. Ole caused apoptosis in breast [
25] and colon tumors at 400 and 800 µM [
31] but not in U251 and A172 in in vitro glioma cancer cells at 200 or 400 µM [
45]. Ole (200 µM) enhanced apoptosis in in vitro MIA PaCa-2 pancreatic cancer cells via the dimerization of c-Jun and c-Fos into API [
59]. In addition to Bax/Bcl2, Ole (200 µM) promoted apoptosis in in vitro MIA PaCa-2 pancreatic cancer cells via the dimerization of c-Jun and c-Fos into AP1 [
59]. In in vitro, Ole promoted cell apoptosis in TCAM-2 and SEM-1 cells by concomitantly enhancing the pro-apoptotic potential in these cells by the overexpression of BAX [
30].
Another important mechanism in which Ole has a role in apoptosis is the p38 MAPK pathway [
57]. One study found that Ole’s (200 µM) apoptotic ability is mediated by the p38/ATF-2 pathway in in vitro NSCLC H1299 lung cancer [
57]. Similar results were seen in MCF-7 breast cancer [
25], Hela cervical cancer cell [
25], in vitro A549 lung cancer (200 µM) [
60], and in vitro neuroblastoma SH-SY5Y [
55] cell lines. However, treatment with Ole at doses of 200 or 400 in glioma cancer cells in vitro had no effect on the p38, ERK, or Jun N-terminal kinase (JNK) pathways [
45].
2.3. Antioxidant Properties of Ole
The antioxidant effects of Ole and its derivatives are mediated by the breakdown of the radical chain [
67]. Ole (100 µmol/L dissolved in dimethyl sulfoxide) showed partial antioxidant and pro-oxidant characteristics in HepG2 hepatocarcinoma cells in vitro, with no harmful effects, according to one study. The activation of reactive oxygen species (ROS) is responsible for this pro-oxidant property [
15]. Furthermore, the antioxidant impact of Ole is dependent on the cell type, time, and concentration of exposure [
15,
59]. Antioxidant effects were identified in non-malignant tissues; however, pro-oxidant stimulating ROS activation was seen in cancer tissue such as breast, prostate, and leukemia HL-60 cells [
15]. Furthermore, a concentration larger than 100 µmol/l caused cell death, whereas a concentration less than that had an antioxidant effect [
15]. In vitro, Ole (500 µM) possesses antioxidant and pro-oxidant effects on non-tumorous BPH-1 prostate cells as well as LNCaP and DU145 tumor cells [
52]. Furthermore, an increase in the heme-oxygenase 1 (HO-1) enzyme at doses of 100 and 500 µM, which is a potent antioxidant containing thiol groups, is thought to be the mechanism by which the antioxidant action is exclusive to BPH-1 cells [
52]. Several antioxidants, including glutathione (GSH) and others, need the presence of thiol groups [
70]. Furthermore, rather than a change in GSH production via glutamyl cysteine synthetase (GCS) expression, the antioxidant impact in the in vitro prostate DU145 cell line is likely to be associated with a reduction in ROS [
52]. According to certain studies, Ole’s antioxidant action has a chemo-protective effect, as evidenced by the fact that it slows colon cancer progression [
16]. In in vitro TPC-1 and BCPAP thyroid cancer cell lines, the antioxidant effect of Ole and peracetylated Ole at concentrations of 100 µM was also detected [
49]. Ole (as a dissolved solution in culture conditions) is thought to minimize oxidative stress by modulating intracellular GSH, a powerful antioxidant molecule. This was seen in vitro in human glioblastoma cells (U87) by Ole at 10 µM. Furthermore, Ole pre-treatment dramatically reduced NO and inducible nitric oxide synthase iNOS gene expression in these cells [
71], supporting the chemo-protective role. The capacity of ROS to activate the Akt pathway could explain why giving Ole to cancer cells causes cell cycle arrest. Because ROS plays such an important part in cancer [
72], it may potentially play a function in malignancy. Ole promotes ROS production in cancerous tissues while also inhibiting the Akt pathway, as previously mentioned. This dual impact could lead to an increase in intracellular ROS, which could lead to cell arrest [
7,
72].
2.4. Ole and Cell Viability
Breast cancer is divided into three subtypes based on chemotherapeutic sensitivity: (1) estrogen receptor-positive (ER+); (2) overexpressing human epidermal growth factor receptor 2 (HER2+), which can be ER+ or ER− and (3) triple-negative (TN), which lacks estrogen, progesterone, and HER2 receptor expression [
5]. In HER2+ breast cancer cells, Ole aglycone (6.25–100 µM) was observed to reduce cell viability and apoptosis. Reduced extracellular domain cleavage, autophosphorylation, and HER2 expression are all linked to this action [
5]. Ole failed to diminish cell viability in vitro hepatic cancer cells [
15], but it did reduce cell viability in pancreatic cancer cells [
59]. Furthermore, this effect was found to be specific to tumor cells and not to healthy cells [
59]. A study comparing the effects of Ole on ER-negative MDA-MB-231 and ER-positive MCF-7 in in vitro cells found that the former is more responsive to Ole treatment than the latter at concentrations of 100 µM [
28]. Most recently, a study carried out by Bossio et al. demonstrated that Ole (15–200 μM) has an inhibition effect on the cell viability assay in a dose-dependent manner in both intra- and extragonadal TCAM-2 and SEM-1 seminoma cells [
30].
2.5. Ole and Cell Cycle Arrest
The effects of Ole on the cell cycle have been verified in numerous research. Ole (100 µg/mL) was shown to be more effective than its derivative HT (25 µg/mL) in slowing the transition from G1 to S phase in MCF-7 breast cancer cell lines [
5,
26]. Ole also produced a delay in the S phase cell cycle in MDA-MB-231 breast cancer cells in another in vitro investigation by upregulating p21 [
14,
73,
74]. The effect of Ole on the MDA-MB-231 cell cycle was shown to be at the sub-G1 phase [
54].
Ole also slowed mitosis, which is thought to be related to a reduction in COX-2, which interrupts the cell cycle in the G2/M phase. This effect was detected in mice given 150 and 225 mg/kg/day of Ole diluted in distilled water by gastric lavage [
9]. The down-regulation of cyclin-D1, 2 and 3, and CDK4 and 6 gene expression, as well as the up-regulation of p53 [
59] and cyclin-dependent kinase inhibitor gene expression are likely to be responsible for the cell cycle arrest effects of Ole (350 µM) on in vitro neuroblastoma [
55]. In neuroblastoma, Ole has a particular effect on the G1/S phase [
58]. Cyclins and their allosteric activators, cyclin-dependent kinases (CDK), play critical roles in cell cycle control. The overexpression of cyclins, particularly cyclin D1, has been confirmed to have a role in cancer. Cyclin D1 can regulate the G1/S phase of the cell cycle via retinoblastoma (Rb) phosphorylation. Pancreatic cancer; non-small cell lung carcinoma; breast, NSCLC, head, and neck squamous cell carcinoma; melanoma; and endometrial, colorectal, and mantle cell lymphoma malignancies all have hyperphosphorylation of Rb [
73].
Furthermore, Ole (200 µM) or its metabolite HT (100 µM) suppresses the cell cycle in the G2 phase in MIA PaCA-2 pancreatic cancer cells in vitro [
59].
2.6. Ole as a Cytoskeleton Disruptor
Ole disrupted actin filaments in the cytoskeleton of breast cancer cells (MCF-7) within two hours in vivo. Interestingly, when Ole was combined with D-glucose, the potential of Ole to disrupt the cytoskeleton was reduced, suggesting the involvement of the glucose transporters (GLUTs) [
76]. Ole’s capacity to cause cell rounding in ovarian cancer cells is attributed to the disruption of the actin cytoskeleton, which hinders these cells from replicating and invasiveness [
14].
2.7. Ole and Fatty Acid Synthase
Ole’s anti-cancer benefits could be attributed to its capacity to inhibit the fatty acid synthase enzyme (FASN), which could be linked to the fact that Ole modifies gene expression and enzymatic activity of this enzyme, as seen in colon cancer cell lines SW620 and HT-29 in vitro [
16]. FASN is overexpressed in a variety of cancers, including prostate, ovarian, breast, endometrial, thyroid, colorectal, bladder, lung, thyroid, oral, tongue, esophageal, hepatocellular, pancreatic, and gastric carcinomas; malignant melanoma; mesothelioma; nephroblastoma; and retinoblastoma; as well as soft tissue sarcoma [
77]. In tumor cells, the overexpression of this enzyme impacts mitochondrial activity as well as peroxisomes, nuclei, and endoplasmic reticula. Furthermore, unlike healthy cells, cancers use fatty acid in de novo synthesis regardless of circulating lipid levels [
78].
2.8. Ole and Inflammation
Inflammation is a condition that occurs in injured cells and is characterized by the production of pro-inflammatory cytokines such as IL-6 and IL-1, as well as the activation of COX and iNOS [
1]. The activation of the NF-kB pathway by these cytokines, as well as the overexpression of MMP are all part of the inflammation process [
1]. There is a well-established relationship between chronic inflammation and cancer progression and development. At various phases of cancer progression, all immune cells are implicated. This persistent inflammation as well as the proinflammatory cytokines IL-1 and IL-6 promote tumor formation in lung and breast cancer models [
42]. Many proinflammatory cytokines, including IL-6, IL-8, IL-1, and TNF-, have been identified as altering bone tumor angiogenesis, progression, and tumor microenvironment [
41]. One of the most important properties of a premetastatic niche is inflammation [
45]. Ole aglycone (10 µM for 5 weeks) suppresses the establishment of a premetastatic niche by lowering the release of IL-8 and MMP in senescence-associated secretory-phenotype cells [
1].
This entry is adapted from the peer-reviewed paper 10.3390/life12081140