Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As a member of the interleukin-1 receptor superfamily, the protein ST2 (alternative name for “interleukin-1 receptor-like 1”) presents with a transmembrane (ST2L) and soluble (sST2) isoforms. Since the discovery of interleukin-33 (IL-33) in 2005 as the ST2L ligand, the molecule has been deeply considered in inflammatory conditions, fibroproliferative diseases, autoimmune diseases, trauma, sepsis, and most recently and significantly in pulmonary and cardiovascular diseases.
- biomarkers
- sST2
- acute heart failure
1. The Molecular Setting
Localized on chromosome 2 (2q12), the ST2 gene encodes different isoforms through alternative promoter splicing. In particular, the transmembrane (ST2L) and the soluble (sST2) ones are particularly important in the inflammation and fibrosis processes [5].
1.1. IL-33/ST2L Axis Signaling
ST2L is expressed through several cells (basophiles, CD4 lymphocytes, eosinophils, macrophages, and keratinocytes) and its role is tightly linked to the release of its natural ligand, the interleukin-33 (IL-33). Nowadays it is usual to consider the IL-33/ST2L axis to the full comprehension of the inflammatory cytokines/chemokines production and the successive regulation of innate and adaptive immune systems to promote inflammatory responses. Historically, the ST2 (also called the IL-33 receptor, T1, DER4, and FIT-1) was described in advance of the detection of IL-33, with potential confusion in terminology. ST2 was originally cloned as an oncogene-induced gene from murine fibroblasts and later a second similar ST2 mRNA transcript was detected and predicted to code for a receptor, now identified as the transmembrane-bound ST2L receptor [6]. The first molecule identified was the secreted isoform and it is now designated an sST2 [7].
IL-33, identified by Schmitz et al. in 2005 [1], belongs to the Toll-like/IL-1-receptor superfamily and can be considered an alarmin which signals tissue damage. In fact, it is secreted by most cells in response to exposure to pathogens, injury-induced stress, or death by necrosis [8]. Its expression has been observed in many organs such as stomach, lung, spinal cord, brain, skin. The strongest expression was found in endothelial cells, epithelial cells, keratinocytes, fibroblasts, fibrocytes and smooth muscle cells [9]. The action of IL-33 is exerted through two mechanisms: as a nuclear factor that directly binds to chromatin in the nucleus and as a cytokine joining to ST2L.
In physiologic conditions, the intracellular localization of IL-33 is mainly in the nucleus, where it regulates gene expression in several ways; particularly as a direct action in suppressing the NF-kB-regulated genes that are necessary for pro-inflammatory signaling [10] but also as an epigenetic modulator via histone deacetylase-3 (HDAC3) [11].
In pathological conditions such as in the necrosis processes, IL-33 reveals its “alarmin” nature and it is released by the cell in the extracellular space. By binding to the ST2L receptor, IL-33 exerts its cellular functions through several signal pathways. In particular, the molecular function is obtained by the action of the heterodimeric ST2L/IL-1RAcP complex on a variety of immune cells, where the IL-1R accessory protein (IL-1RAcP) plays an essential role by enhancing the affinity of IL-33 for ST2L.
In more details, the binding of the IL-33 to ST2L allows the receptor to experience a conformational change, which enables the recruitment of IL-1RAcP [7]. Heterodimerization of the two transmembrane molecules conveys the two intracellular TIR domains together. Successive receptor adaptor proteins are recruited, and the signal is transmitted by the adaptor proteins myeloid differentiation factor-88 and IL-1R-Associated Kinase (MyD88-IRAK1), respectively, and the 4-TRAF6 signaling pathway with resulting degradation of the inhibitory protein IκB and subsequent activation of the NF-κB transcription factor [1]. Furthermore, MAP kinases, p38, JNK, and ERK are also activated with triggering of downstream transcription factors, such as AP-1, that enables transcription of cytokines and chemokines in a cell-type restricted manner.
Activation of this pathway in Th2 cells leads to production of Th2 cytokines (i.e., IL-4, IL-5, IL-13) [1], whereas in epithelial cells, ST2 activation by IL-33 results predominantly in chemokine activation [12].
During wound healing, extracellular IL-33 interacts with the ST2L receptor and the complex of IL-33/ST2L activates myeloid differentiation primary response 88 (MyD88) intracellular cascades that drive production of type 2 cytokines (such as IL-13) from polarized Th2 cells. Most recently, tissue fibrosis, mucosal healing, and wound repairment were found to be additional possible actions of IL-33 during inflammation processes.
1.2. IL-33/sST2 Molecular Role
The second variant of the ST2 gene expression is the circulating soluble ST2 (sST2), which is shorter than ST2L. It is identical in structure to the extracellular region of the long ST2L isoform except for nine additional amino acids at the C-terminus and it is obtained [3,13] through differential mRNA processing. It can be produced spontaneously in the lung, kidney, heart, and small intestine and after activation with IL-33 in mast cells or with anti-CD3/anti- CD28 in both CD4 and CD8 T cells. Its expression is largely inducible and ubiquitous in living cells. The role of sST2 was recently investigated as an IL-33 soluble receptor and blocker of effects in target cells [1]. Specifically, sST2 avidly binds to IL-33 and prevents its fastening to ST2L in the immune cell (mainly lymphocytes T). In this framework, sST2 can be thought of as a decoy receptor. As a consequence, it can inhibit the activation of Th2 cell response and the release of anti-inflammatory cytokines (IL-4, IL-5, IL-10, IL-13), polarizing the Th1 response, which results in the activation and release of inflammatory cytokines (TNF-α) and inflammation.
Figure 1 provides a sketch of the two different isoforms ST2d and sST2 with the possible biological actions.
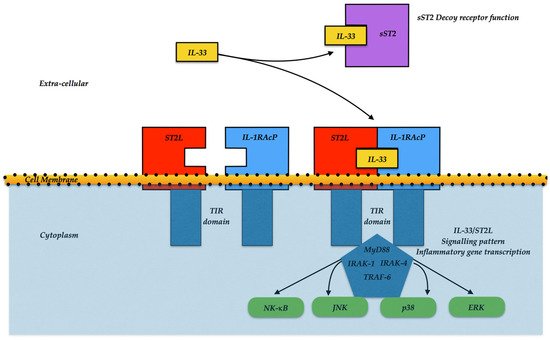
Figure 1. IL-33/ST2L signaling pattern. IL-33 can bind to the ST2/IL-1 receptor accessory protein (IL-1RAP) heterodimer, then enroll MyD88 to its intracellular domain. Alternatively, the IL-33 binds to the sST2 decoy receptor, impairing the further signal. MyD88 fastening involves IL-1R-associated kinase (IRAK) and TRAF6, leading to either the NF-κB, JNK, p38, or ERK activation, and promoting inflammatory cytokine expressions.
2. The Clinical Setting
2.1. Acute Heart Failure
While pro-Brain Natriuretic Peptide (pro-BNP) is widely recognized as the gold standard marker for the diagnosis of acute heart failure (HF) in routine clinical practice, several studies have evaluated the use of different markers with potential prognostic value in HF patients; novel molecules such as sST2, emerge as potentially useful biomarkers, providing additional diagnostic and prognostic value with different and controversial findings in relation to the studied population and the end points evaluated. The American Heart Association/American College of Cardiology guidelines on the management of HF recommend the measurement of sST2, in addition to other fibrosis biomarkers, in patients with acute HF for a more appropriate stratification [14]; moreover, sST2, unlike NT-proBNP, is not influenced by age, body mass index (BMI), renal function, or the etiology of HF [15].
In a prospective single-center study, pro-BNP and sST2 showed high diagnostic power for HF (AUC 0.976 and 0.889, respectively), but sST2 revealed stronger power to predict fatal events (in-hospital and 1 month mortality rates), as well as other markers of negative prognosis such as the use of inotropes or high lactate levels [16]. The American heart failure cohort study (PRIDE study) [17] firstly analyzed the role of sST2 in defining acute HF in patients enrolled in emergency department with acute dyspnea, and NT-proBNP was significantly superior to sST2; however, this study suggested that sST2 was more important as a prognostic tool, regarding mortality, due to HF. Regarding this prognostic aspect, sST2 and NT-proBNP showed additive significance; in fact, the increase in both markers showed the highest mortality rate after 1 year and 4 years (about 40%). This study suggested a threshold of sST2 ≥ 35 ng/mL as a predictor for poor prognosis and risk of death [17].
In a large Asian population, Yamamoto M et al. evaluated the additional clinical value of sST2, Pentraxin 3, Galectin-3, and high-sensitive cardiac troponin (hs-TnT) beyond BNP for risk stratification, showing that sST2 was associated with significant outcomes (all-cause, cardiovascular mortality, and HF hospitalization) in patients with acute decompensated HF solely in subjects with preserved ejection fraction [18]. On the other hand, in a large study conducted by Edmin M. et al. [19] in a population with a prominent reduced ejection fraction (more than 80% with reduced left ventricular ejection fraction (LVEF) <40%) in a multivariate model (including age, sex, BMI, ischemic etiology, LVEF, NYHA classification, glomerular filtration rate, medical therapy, NT-proBNP, and hs-TnT), the risk of all-cause death, cardiovascular death, and HF hospitalization were increased by 26%, 25%, and 30%, respectively, per each doubling of sST2 [19]. The same results were obtained in the prospective study by Zhang R. et al.; higher levels of sST2 showed a significantly higher rate of adverse outcomes (all-cause mortality or heart transplantation), strictly correlated to left ventricular ejection fraction [11]. In addition, several multicenter cohort studies (i.e., MOCA study and ASCEND-HF study) showed that sST2 concentration had high ability in predicting CV mortality at short term (30 days) as well as long term (1 year) [20,21].
In the prospective cohort study, Wang Z. et al. evaluated 331 patients affected by acute HF according to sST2 levels, and followed progression for almost 2 years; patients with higher behaviors of sST2 had a higher left ventricular mass index, lower left ventricular ejection fraction, higher NYHA score and higher NT-proBNP levels; moreover this group showed the worst primary outcome (cardiovascular mortality) in all patients with acute AF [22].
The Oulu Project Elucidating Risk of Atherosclerosis (OPERA) Survey collected data during a long-term follow-up (21 years), evaluating several data about morbidity and mortality. The main determinant of sST2 levels during the follow-up was smoking habit, and diabetes in the multivariate model male sex; levels of alanine aminotransferase (ALAT), high-density lipoprotein (HDL) cholesterol, and high-sensitive C-Reactive Protein (hsCRP) were associated with elevated sST2 levels. Moreover, sST2 levels were higher among subjects suffering from cardiovascular disease, cancer, mild cognitive decline, and diabetes. ST2 was found as an independent predictor of total mortality, evaluated with several covariates (age, sex, diabetes, smoking, transaminases, HDL-cholesterol, and hsCRP) [23].
Conversely, in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) cohort, sST2 was significantly associated with secondary endpoints (worsening HF and hospitalization due to worsening HF) in older patients with chronic HF, whereas sST2 did not show prognostic significance regarding primary endpoint (CV death, non-fatal myocardial infarction, or stroke) pro-BNP, and C-reactive protein [24].
Interestingly, a significant aspect resides in the variation in sST2 levels during the treatment of HF; in fact, more recent studies claim that continuous measurements greatly increase the amount of available prognostic information, and the use of sST2 as a monitoring tool [2]. The Prospective Randomized Amlodipine Survival Evaluation 2 (PRAISE-2) study conducted on severe chronic heart failure (NYHA class III to IV) suggested that the variation in sST2 during hospitalization, more than baseline sST2 value, is a significant predictor of end-point, beyond well-known predictive significance of baseline BNP and baseline precursor peptide of atrial natriuretic peptide (ProANP) [2]. In a meta-analysis conducted on a large population that was followed for more than 1 year, Aimo A et al. showed that both values of sST2 obtained at admission and at discharge from hospitalization had significant prognostic value regarding all-cause and CV deaths, but sST2 at discharge was more predictive of HF re-hospitalization during the follow-up (HR higher than 2.5 times) [25].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23158230
This entry is offline, you can click here to edit this entry!
