Maternal milk, a complex fluid with several bioactive factors, is the best option for the newborn. Its dynamic composition is influenced by diverse factors such as maternal age, lactation period, and health status.
- adipokines
- antioxidants
- breastfeeding
- cytokines
- growth factors
1. Introduction
2. Bioactive Compounds in Breastmilk
2.1. Antioxidants in Human Milk
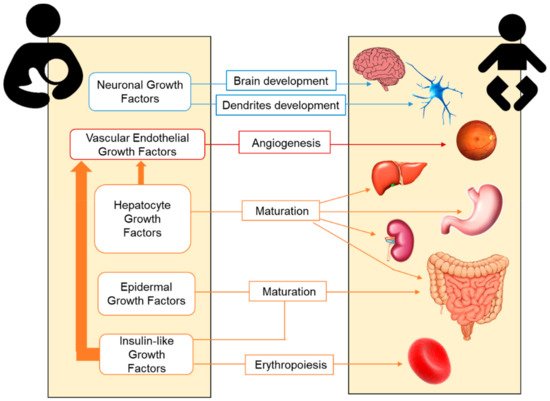
2.2. Growth Factors in Human Milk
2.3. Adipokines in Human Milk
|
Adipokines |
Tissue Synthesized |
Range (ng/mL) |
Preterm Infants |
Main Neonatal Functions |
|---|---|---|---|---|
|
Leptin |
White adipose Placenta Mammary |
0.2–10.1 |
↑/? |
Anorexigenic T-lymphocyte responses |
|
Adiponectin |
Adipocytes |
4.2–87.9 |
≈/? |
Orexigenic Regulation of lipid/glucose metabolism Improvement of insulin sensitivity Anti-inflammatory actions |
|
Resistin |
Immune cells Epithelial cells |
0.2–1.8 |
↑/? |
Regulation of glucose homeostasis Inhibition of adipocyte differentiation Inflammatory response |
|
Ghrelin |
Stomach Pituitary Other |
0.07–6 |
? |
Orexigenic Gastric motility and secretion Adipogenesis Anti-inflammatory actions |
|
Obestatin |
Stomach Small intestine |
0.4–1.3 |
? |
Anorexigenic Body weight regulation |
|
Nesfatin |
Neurons Pancreas Other |
0.008–0.01 |
? |
Anorexigenic Production of body fat |
|
Apelin |
Heart Lung Other |
43–81 |
? |
Regulation of cardiovascular system Fluid homeostasis Angiogenesis Regulation of insulin secretion |
Adapted from Catli et al. (2014) [47]. Arrows indicate the comparison with term infants (↑, higher; ↓, lower; ≈, similar); ? indicates unavailable or inconclusive data and the need for research.
2.4. Cytokines in Human Milk
3. Human Milk Cells
3.1. Stem Cells in Human Milk
3.2. Leukocytes in Human Milk
4. Human Milk Microbiota
This entry is adapted from the peer-reviewed paper 10.3390/nu11061307
References
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94.
- World Health Organization: Preterm Birth. Available online: https://bit.ly/2RWokG3 (accessed on 23 November 2018).
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.; Kinney, M. Born Too Soon: The Global Epidemiology of 15 Million Preterm Births. Reprod. Health 2013, 10, 1–14.
- Harrison, M.S.; Goldenberg, R.L. Global Burden of Prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79.
- Iams, J.D.; Romero, R.; Culhane, J.F.; Goldenberg, R.L. Primary, Secondary, and Tertiary Interventions to Reduce the Morbidity and Mortality of Preterm Birth. Lancet 2008, 371, 164–175.
- Ion, R.; López Bernal, A. Smoking and Preterm Birth. Reprod. Sci. 2015, 22, 918–926.
- Al-gubory, K. Environmental Pollutants and Lifestyle Factors Induce Oxidative Stress and Poor Prenatal Development. Reprod. Biomed. Online 2014, 29, 17–31.
- Simón, L.; Pastor-Barriuso, R.; Boldo, E.; Fernández-Cuenca, R.; Ortiz, C.; Linares, C.; Medrano, M.J.; Galán, I. Smoke-Free Legislation in Spain and Prematurity. Pediatrics 2017, 139, e20162068.
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, Regional, and Worldwide Estimates of Preterm Birth Rates in the Year 2010 with Time Trends since 1990 for Selected Countries: A Systematic Analysis and Implications. Lancet 2012, 379, 2162–2172.
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding Strategies for Premature Infants: Beneficial Outcomes of Feeding Fortified Human Milk Versus Preterm Formula. Pediatrics 1999, 103, 1150–1157.
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of Human Milk in Extremely Low Birth Weight Infants’ Risk of Necrotizing Enterocolitis or Death. J. Perinatol. 2009, 29, 57–62.
- Maayan-Metzger, A.; Avivi, S.; Schushan-Eisen, I.; Kuint, J. Human Milk Versus Formula Feeding Among Preterm Infants: Short-Term Outcomes. Am. J. Perinatol. 2012, 29, 121–126.
- Reiterer, F.; Scheuchenegger, A.; Resch, B.; Maurer-Fellbaum, A.; Avian, A.; Urlesberger, B. Outcomes of Very Preterm Infants with and without BPD Followed to Preschool Age. Pediatr. Int. 2019, 61, 381–387.
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of Breast Milk on Intelligence Quotient, Brain Size, and White Matter Development. Pediatr. Res. 2010, 67, 357–362.
- Parkinson, J.R.C.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm Birth and the Metabolic Syndrome in Adult Life: A Systematic Review and Meta-Analysis. Pediatrics 2013, 131, e1240–e1263.
- Lapillonne, A.; Griffin, I.J. Feeding Preterm Infants Today for Later Metabolic and Cardiovascular Outcomes. J. Pediatr. 2013, 162, S7–S16.
- Howson, C.P.; Kinney, M.V.; Mcdougall, L.; Lawn, J.E. Born Too Soon: Preterm Birth Matters. Reprod. Health 2013, 10, 1–9.
- Castillo-Castañeda, P.C.; Gaxiola-Robles, R.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Defensas Antioxidantes En Leche Materna En Relación Al Número De Gestas Y A La Edad De Las Madres. Nutr. Hosp. 2014, 30, 540–547.
- Castillo-Castañeda, P.C.; García-González, A.; Bencomo-Alvarez, A.E.; Barros-Nuñez, P.; Gaxiola-Robles, R.; Celina Méndez-Rodríguez, L.; Zenteno-Savín, T. Micronutrient Content and Antioxidant Enzyme Activities in Human Breast Milk. J. Trace Elem. Med. Biol. 2019, 51, 36–41.
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74.
- World Health Organization: Exclusive Breastfeeding. Available online: https://bit.ly/2FaaEkE (accessed on 23 November 2018).
- World Health Organization: Infant and Young Child Feeding. Available online: https://bit.ly/2VyOnRL (accessed on 5 March 2019).
- American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841.
- Singhal, A.; Sadaf Farooqi, I.; O’Rahilly, S.; Cole, T.J.; Fewtrell, M.; Lucas, A. Early Nutrition and Leptin Concentrations in Later Life. Am. J. Clin. Nutr. 2002, 75, 993–999.
- Lessen, R.; Kavanagh, K. Practice Paper of the Academy of Nutrition and Dietetics Abstract: Promoting and Supporting Breastfeeding. J. Acad. Nutr. Diet. 2015, 115, 450.
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and Antioxidant Status of Human Breast Milk During Lactation Period. Dairy Sci. Technol. 2015, 95, 295–302.
- Singhal, A.; Cole, T.J.; Lucas, A. Early Nutrition in Preterm Infants and Later Blood Pressure: Two Cohorts after Randomised Trials. Lancet 2001, 357, 413–419.
- Cubero, J.; Sánchez, C.L.; Bravo, R.; Sánchez, J.; Rodriguez, A.B.; Rivero, M.; Barriga, C. Analysis of the Antioxidant Activity in Human Milk, Day Vs. Night. Cell Membr. Free Radic. Res. 2009, 1, 100–101.
- De Vicente, B. 1 De Cada 5 Bebés no Recibe Leche Materna En Los Países Ricos. Available online: https://bit.ly/2RiYzeR (accessed on 21 January 2019).
- Heiman, H.; Schanler, R.J. Nutrición Enteral En Prematuros: El Rol De La Leche Humana. Rev. Enferm. 2007, 12, 26–34.
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681.
- Mehta, R.; Petrova, A. Is Variation in Total Antioxidant Capacity of Human Milk Associated with Levels of Bio-Active Proteins? J. Perinatol. 2014, 34, 220–222.
- Savino, F.; Benetti, S.; Liguori, S.A.; Sorrenti, M.; Montezemolo, L.C. Di Advances on Human Milk Hormones and Protection Against Obesity. Cell. Mol. Biol. 2013, 59, 89–98.
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896.
- Mutinati, M.; Pantaleo, M.; Roncetti, M.; Piccinno, M.; Rizzo, A.; Sciorsci, R.L. Oxidative Stress in Neonatology: A Review. Reprod. Domest. Anim. 2014, 49, 7–16.
- Wilinska, M.; Borszewska-Kornacka, M.K.; Niemiec, T.; Jakiel, G. Oxidative Stress and Total Antioxidant Status in Term Newborns and Their Mothers. Ann. Agric. Environ. Med. 2015, 22, 736–740.
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxid. Med. Cell. Longev. 2018, 2018, 1–7.
- Thibeault, D.W. The Precarious Antioxidant Defenses of the Preterm Infant. Am. J. Perinatol. 2000, 17, 167–182.
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants During Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175.
- Shelby, R.D.; Cromeens, B.; Rager, T.M.; Besner, G.E. Influence of Growth Factors on the Development of Necrotizing Enterocolitis. Clin. Perinatol. 2019, 46, 51–64.
- Lawrence, R.M.; Pane, C.A. Human Breast Milk: Current Concepts of Immunology and Infectious Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2007, 37, 7–36.
- Loui, A.; Eilers, E.; Strauss, E.; Pohl-Schickinger, A.; Obladen, M.; Koehne, P. Vascular Endothelial Growth Factor (VEGF) and Soluble VEGF Receptor 1 (sFlt-1) Levels in Early and Mature Human Milk from Mothers of Preterm versus Term Infants. J. Hum. Lact. 2012, 28, 522–528.
- Hirai, C.; Ichiba, H.; Saito, M.; Shintaku, H.; Yamano, T.; Kusuda, S. Trophic Effect of Multiple Growth Factors in Amniotic Fluid or Human Milk on Cultured Human Fetal Small Intestinal Cells. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 524–528.
- Munblit, D.; Abrol, P.; Sheth, S.; Chow, L.Y.; Khaleva, E.; Asmanov, A.; Lauriola, S.; Padovani, E.M.; Comberiati, P.; Boner, A.L.; et al. Levels of Growth Factors and Iga in the Colostrum of Women from Burundi and Italy. Nutrients 2018, 10, 1216.
- Saso, A.; Blyuss, O.; Munblit, D.; Faal, A.; Moore, S.E.; Doare, K. Le Breast Milk Cytokines and Early Growth in Gambian Infants. Front. Pediatr. 2019, 6, 414.
- Savino, F.; Liguori, S.A.; Lupica, M.M. Adipokines in breast milk and preterm infants. Early Hum. Dev. 2010, 86, 77–80.
- Catli, G.; Anik, A.; Tuhan, H.Ü.; Kume, T.; Bober, E.; Abaci, A. The relation of leptin and soluble leptin receptor levels with metabolic and clinical parameters in obese and healthy children. Peptides 2014, 56, 72–76.
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508.
- Gregory, K.E.; Walker, W.A. Immunologic factors in human milk and disease prevention in the preterm infant. Curr. Pediatr. Rep. Online 2013, 1, 222–228.
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186.
- Watanabe, M.A.E.; de Oliveira, G.G.; Oda, J.M.M.; Ono, M.A.; Guembarovski, R.L. Cytokines in Human Breast Milk: Immunological Significance for Newborns. Curr. Nutr. Food Sci. 2012, 8, 2–7.
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605.
- Peroni, D.G.; Pescollderungg, L.; Piacentini, G.L.; Rigotti, E.; Maselli, M.; Watschinger, K.; Piazza, M.; Pigozzi, R.; Boner, A.L. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr. Allergy Immunol. 2010, 21, 977–982.
- Rajani, P.S.; Seppo, A.E.; Järvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front. Pediatr. 2018, 6, 6.
- Polat, A.; Tunc, T.; Erdem, G.; Yerebasmaz, N.; Tas, A.; Beken, S.; Basbozkurt, G.; Saldir, M.; Zenciroglu, A.; Yaman, H.; et al. Interleukin-8 and its receptors in human milk from mothers of full-term and premature infants. Breastfeed. Med. 2016, 11, 247–251.
- Patki, S.; Kadam, S.; Chandra, V.; Bhonde, R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum. Cell 2010, 23, 35–40.
- Molès, J.P.; Tuaillon, E.; Kankasa, C.; Bedin, A.S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143.
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8, 584.
- Järvinen, K.-M.; Soumalainen, H. Leucocytes in human milk and lymphocyte subsets in cow ’s milk-allergic infants. Pediatr. Allergy Immunol. 2002, 13, 243–254.
- Vass, R.A.; Kemeny, A.; Dergez, T.; Ertl, T.; Reglodi, D.; Jungling, A.; Tamas, A. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 2019, 14, 1–10.
- Bendiks, M.; Kopp, M.V. The relationship between advances in understanding the microbiome and the maturing hygiene hypothesis. Curr. Allergy Asthma Rep. 2013, 13, 487–494.
- Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, e724–e732.
- Mcguire, M.K.; Mcguire, M.A. Human Milk: Mother Nature’s Prototypical Probiotic Food? Adv. Nutr. 2015, 6, 112–123.
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front. Microbiol. 2016, 7, 3389.
- Munblit, D.; Peroni, D.G.; Boix-Amorós, A.; Hsu, P.S.; Land, B.V.; Gay, M.C.L.; Kolotilina, A.; Skevaki, C.; Boyle, R.J.; Collado, M.C.; et al. Human Milk and Allergic Diseases: An Unsolved Puzzle. Nutrients 2017, 9, 894.
- Grönlund, M.; Gueimonde, M.; Laitinen, K.; Kociubinski, G.; Gronroos, T.; Salminen, S.; Isolauri, E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 2007, 37, 1764–1772.
- Martín, V.; Maldonado-Barragán, A.; Moles, L.; Rodriguez-Baños, M.; Del Campo, R.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Sharing of Bacterial Strains Between Breast Milk and Infant Feces. J. Hum. Lact. 2012, 28, 36–44.
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of maternal consumption of Lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170.
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 1–14.
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.A.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677.
