4. Behavior, Cognition, and Emotion
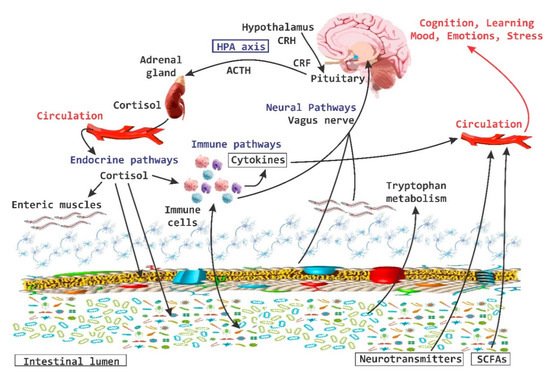
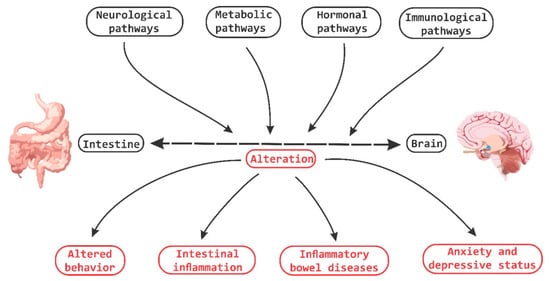
It has been demonstrated that bi-directional communication exists between the intestine and the brain and that it involves neurological, metabolic, hormonal and immunological signaling pathways; and that disturbance or alteration in these systems can result in altered behavior [
83]. A clear example is intestinal inflammation, which has been associated with changes in bowel-brain interactions, as well as a high morbidity between inflammatory bowel disorder and anxiety states (
Figure 4) [
84].
Figure 4. Brain-Gut Homeostasis. The relationship between the intestine and the brain involves signaling pathways at a neural, metabolic, hormonal and immune system levels. The alteration in these pathways is capable of causing changes in cognitive and behavioral processes, as well as inducing inflammatory processes at the periphery level.
The role of microbiota has not only focused on the impact it exerts on the brain and central nervous function but also on how it is intimately related to the constitutive modulation of nerve function at the peripheral central level [
71].
Stress has been defined as a very complex dynamic condition in which homeostasis or the internal “resting state” is altered or threatened [
85,
86]. Throughout life all organisms are exposed to factors that exceed the homeostatic threshold, which results in a stress response, which may be physical, psychological or immunological. Evolution has armed most organisms with the necessary biological machinery to mount a defense response to acute stressors and restore the homeostatic balance once the stress or damage has subsided [
85].
A significant number of animal studies provide abundant evidence that the medial prefrontal cortex (MPFC) plays an important role in the regulation of stress circuitry [
28]. While the ventral part of the MPFC has been augmented with a stimulatory role, the more dorsal part in contrast has been described to possess an activity of HPA-axis inhibition. It has been also described that this negative feedback mechanism is mediated by the inhibition of glucocorticoid receptors (GRs) in the MPFC [
28]. The amygdala is a key region in the process of stress responses in addition to being an important target for the inhibitory feedback system by the MPFC [
87]. In humans, the MPFC area is involved in the modulation of amygdala activity during emotional conflicts and in the regulation of autonomic and affective responses [
28,
88].
Stress, particularly in the early stages of life, is one of the major predictors of the onset of major depression disorder (MDD) [
89]. Early exposure to stress and MDD is associated with a significant de-regularization of the HPA-axis and the stress/cortisol response system. Exposure to stressors, HPA-axis deregulation, elevated corticosteroid levels and major depression states are related to structural alterations in the hippocampus and amygdala, key regions in the regulation of the HPA-axis [
90,
91].
In one study of early life maternal separation, a group of male rats were submitted to stress tests [
79]. They all showed the typical pattern: poor forced swim performance while the group that was separated also showed records of high IL-6 blood levels, low NE levels in brain and higher expression of CRF gene in the amygdala [
92]. By administering
L. rhamnosus R011 plus
L. helveticus R0052, the rats downregulated their HPA axis and normalized their corticosterone levels [
16,
92].
Psychobiotics are now considered key elements in affective disorders. In one experiment with mice that were administered with
L. rhamnosus, they featured lesser signs of anxiety and depression in forced swim and plus elevated maze respectively than their control counterparts, even at the same levels of corticosterone [
16,
93]. This suggests that the probiotic had a downregulation effect over HPA axis [
93]. In the presence of
L. rhamnosus, mice showed a lower hippocampal expression of the GABA
B1b receptor gene and a higher expression of it in the cingulated cortex and limbic regions. Since GABA is the main inhibitory neurotransmitter of the nervous system, it would appear that psycobiotics are able to modulate the local balance of inhibition/exciting in order to control the systemic responses to stress, anxiety and depression [
93].
As previously described, GF-mice exhibit an exaggerated response to stressors, with the presentation of anxious-type behaviors and cognitive deficits [
94,
95]. This behavior is influenced by the amygdala and the hippocampus. The signaling between the basolateral amygdala (BLA) and the ventral hippocampus modulates anxiety behaviors and social behaviors [
96]. Tune changes (structural changes) in the amygdala and hippocampus are associated with anxiety disorders in humans and in rodents in early stages of development. There is evidence of hypertrophy of the dendrites of excitatory neurons in the BLA area under a state of repeated (repetitive) stress that induces atrophy of the dendrites in hippocampal neurons [
94].
The “germ-free” status induces dendritic hypertrophy in inhibitory interneurons, and the excitatory pyramidal neurons of the BLA area show increased density of spines type: “thin”, “stubby” and “mushroom”. The absence of intestinal microbiota induces dendritic atrophy in other areas of the CNS, as is the case of hippocampal pyramidal neurons and granular cells of the dentate gyrus. In GF-animals, there is a significant loss of “stubby” and “mushroom” spines in hippocampal pyramidal neurons [
94].
It has been estimated that there are 32% fewer synaptic connections in hippocampal pyramidal neurons of GF-animals when the dendrite size decreases and this is combined with a smaller size in the same dendritic spines [
94].
A characteristic shared by the animal models of autism and GF-mice is an important alteration in the processes of social behavior. This type of alterations is in turn associated with alterations in the volume of the hippocampus and the amygdala. Changes in the size of these structures have been well documented in experiments with rodents, subject to severe stressors. Prenatally stressed rats experience an increase in the volume of the lateral amygdala [
97,
98] whereas chronic stress or treatment with corticosteroids induces hippocampal atrophy [
98]. Changes in these structures of the CNS are frequently observed in human patients with anxiety disorders or with autism, clearly indicating that the volumetric alterations of the limbic structures can in turn be the result of a maladaptive response to stress [
94]. In chronically stressed mice, dendrite hypertrophy is observed in inhibitory GABAergic neurons of the prefrontal cortex area [
99].
The amygdala has different “target” areas that are responsible for modulating neuroendocrine responses to stress. The BLA area is activated by psychological stressors, and lesions in this area significantly reduce the HPA-axis response efficiency [
94]. While, on the other hand, the area of the central nucleus of the amygdala (CeA) is not involved in the signaling of the HPA-axis induced by stressors, it is an area that also regulates autonomic responses to stress [
94]. GF-mice have a lower degree of anxiety and social cognitive deficit, and it has been mentioned previously that there is an important relationship between anxiety and social behavior; the amygdala and the area of the ventral hypothalamus are directly involved in the regulation of this type of behavior [
100]. In addition to having a preponderant role in the regulation of anxiety, the ventral hypothalamus is also involved in processes of sociability, and an alteration or damage in this area leads to the appearance of abnormal responses to social situations [
101]. Besides, this ventral hippocampus exhibits a very important reciprocal connection with the amygdala, another area involved in anxiety and sociability [
100].
The different tonsillar sub-regions have different roles in the regulation of anxiety and social behavior. The areas of the lateral amygdala (LA) and the BLA area integrate sensory information and adverse situations and then send their projections to the CeA area [
100]. The stimulation of the projections from the BLA to the CeA area induces an anxiolytic phenotype in mice [
102]. This is in contrast to direct stimulation of the entire BLA area, where an opposite effect is generated, suggesting that most of the BLA neurons project towards areas that regulate anxiogenic effects [
102].
It has been mentioned that chronic stress in the adult stage is also capable of affecting the composition of the gut microbiota [
11]. It is clear that alterations in the brain-gut axis interactions are associated with intestinal inflammatory processes, syndromes of chronic abdominal pain, and with eating disorders [
11,
103]. This altered modulation of the brain-gut axis is associated with alterations in the regulation of stress responses and behavioral alterations. The high co-morbidity that exists between stress and some symptoms of psychiatric illnesses such as high anxiety, gastrointestinal disorders (included in irritable bowel syndrome, IBS) is clear evidence of the importance of this axis in the pathophysiology of certain types of diseases [
11].
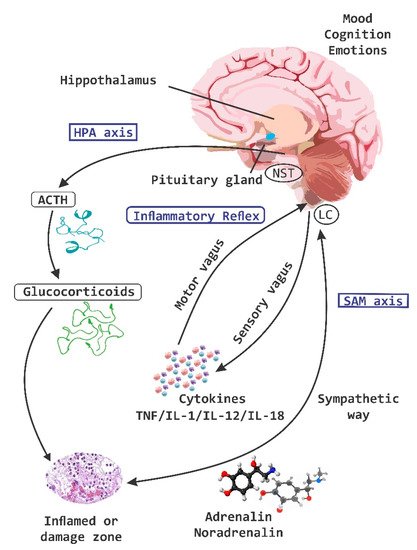
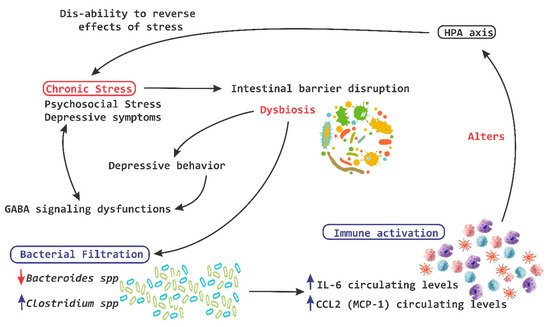
Chronic stress on the other hand breaks the intestinal barrier, causes filtrations and alters the ability of the HPA-axis to reverse the deleterious effects of stress (
Figure 5) [
93,
94].
Figure 5. Chronic stress and HPA axis. A chronic stress process is capable of causing disruption at a level of the intestinal barrier and cause dysbiosis, which in turn induces the leakage of bacteria and the activation of the local immune system, leading to a significant alteration of the hypothalamic pituitary adrenal (HPA)-axis. IL, interleukin; MCP-1, monocyte chemoattractant protein; red arrow down mean decrease levels; blue arrow up mean increase levels.
GABA is the major inhibitory neurotransmitter in the CNS. Dysfunctions in GABA signaling are associated with anxiety and depression [
104]. Lactobacilli and bifidobacteria are able to metabolize glutamate to produce GABA
in vitro [
62,
104,
105]. In an
in vivo experiment in mice, a strain of
Lactobacillus rhamnosus shows an effect and influence on depressive and ancestral behavior, and it can also alter the central expression of GABA receptors in key brain regions for stress management [
62].
In 2006, Kamiya et al. [
106] demonstrated that oral administration of
Lactobacillus species for anesthetized rats is capable of completely suppressing colonic distension induced by pseudo-affective cardiac responses, which is reflected in the inhibition of visceral pain perception. This treatment is also effective in reducing electrical charges in fibers of the dorsal root of the ganglia [
71]. The administration of these same strains of
Lactobacillus to healthy adult rats is enough to activate calcium (Ca
2+) and potassium (K
+) channels in neurons-AH (after hyperpolarization) of the ENS in mesenteric plexus of the colon [
71].
It has been shown that the oral administration of specific strains of
Lactobacillus induces the expression of opioids-μ receptors and cannabinoids and promotes analgesic functions similar to effects of morphine. This suggests that intestinal microbiota can influence our visceral perception [
107]. Altogether, these findings indicate that probiotics are able to modulate the function responsible for the visceral and somatic perception of pain [
71].
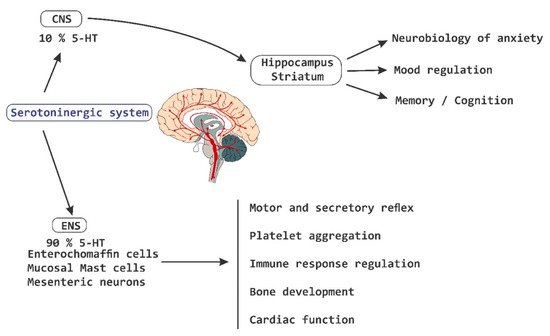
Currently, there is evidence that supports the influence of intestinal microbiota on the behavior and health of SNC [
1]. Patients with depressive symptoms show a significant improvement in the symptoms of depression accompanied by a reduction in plasma TRP after a fructose-restricted diet. Furthermore, fructose malabsorption provides the substrate for a rapid bacterial fermentation, which results in changes in gut motility [
72]. The administration of a strain of
Bifidobacterium infantis for 14 days increases the levels of plasma TRP, suggesting that commensal bacteria have the ability to influence the metabolism of TRP [
93].
Intestinal bacteria are potent regulators of systemic and local immune responses such as that related to mucous membranes, in addition to contributing to the development of inflammatory disorders in the CNS. GF-animals or animals treated with antibiotics with an experimental autoimmune encephalomyelitis (EAE) process present reduced inflammation and a lower degree of disease compared to conventional mice, which suggest the existence of complex interactions between commensal bacteria and the inflammatory process in CNS [
9,
97,
98]. For example, segmented filamentous bacteria (frequently associated with the intestinal epithelium) promote the development of Th17 helper T cells, which produce IL-17. They have been termed as Th17 cells in the small intestine of mice [
99,
108].
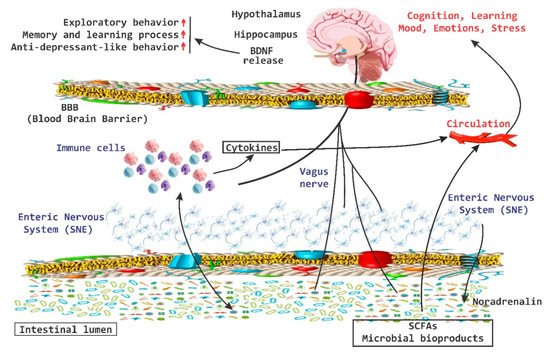
There is important evidence that the brain-gut axis can influence brain chemistry and is able to modulate behavior in adult mice [
43]. A transient disturbance in the microbiota is able to increase the levels of BDNF in the hippocampus, as well as increase the exploratory behavior of animals. In the hippocampus, BDNF is associated with memory and learning processes and recent evidence indicates that this increase is associated with anxiolytic and antidepressant-like behavior [
43]. On the other hand, the amygdala is also associated with memory and disorders in the mood and there has been an increase in the expression of BDNF in the amygdala during processes of “learning fear” [
109]. Low levels of BDNF in the amygdala increase the exploratory behavior of the animals (
Figure 6) [
9,
43].
Figure 6. BDNF release system. The brain-derived neurotrophic factor (BDNF) released via the activation of the brain-gut axis has been associated with cognitive and behavioral processes, as well as with anxiolytic and antidepressive effects. SCFAs, short chain fatty acids; red arrow up mean increase levels.