Iron is an essential trace metal necessary for the reproduction and survival of fungal pathogens. The latter have developed various mechanisms to acquire iron from their mammalian hosts, with whom they participate in a continuous struggle for dominance over iron. Invasive fungal infections are an important problem in the treatment of patients with hematological malignancies, and they are associated with significant morbidity and mortality. The diagnosis of invasive clinical infections in these patients is complex, and the treatment, which must occur as early as possible, is difficult. There are several studies that have shown a possible link between iron overload and an increased susceptibility to infections. This link is also relevant for patients with hematological malignancies and for those treated with allogeneic hematopoietic stem cell transplantation. The role of iron and its metabolism in the virulence and pathogenesis of various invasive fungal infections is intriguing, and so far, there is some evidence linking invasive fungal infections to iron or iron overload. Clarifying the possible association of iron and iron overload with susceptibility to invasive fungal infections could be important for a better prevention and treatment of these infections in patients with hematological malignancies.
- iron
- iron overload
- fungal infection
- hematological malignancies
- iron chelation therapy
1. Invasive Fungal Infections in Hematology
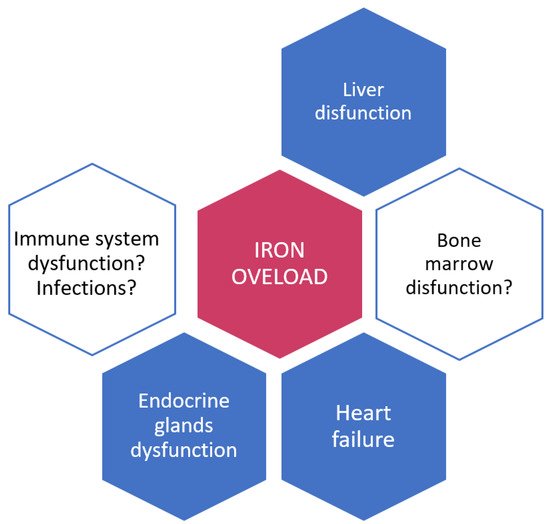
2. Iron and Iron Overload: Their Role in Infections
3. Mechanisms of Iron Acquisition by Fungal Pathogens
3.1. Reduction of Ferric to Ferrous Iron with Subsequent Transport
3.2. Siderophore Production and Transport
This entry is adapted from the peer-reviewed paper 10.3390/jcm11154457
References
- Ninin, E.; Milpied, N.; Moreau, P.; André-Richet, B.; Morineau, N.; Mahé, B.; Vigier, M.; Imbert, B.; Morin, O.; Harousseau, J.; et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin. Infect. Dis. 2001, 33, 41–47.
- Pagano, L.; Caira, M.; Nosari, A.; Van Lint, M.T.; Candoni, A.; Offidani, M.; Aloisi, T.; Irrera, G.; Bonini, A.; Picardi, M.; et al. Fungal infections in recipients of hematopoietic stem cell transplants: Results of the SEIFEM B-2004 study-Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 2007, 45, 1161–1170.
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs fluconazole or intraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359.
- Egerer, G.; Geist, M.J. Posaconazole prophylaxis in patients with acute myelogenous leukemia—Results from an observational study. Mycoses 2011, 54 (Suppl. 1), 7–11.
- Orasch, C.; Weisser, M.; Mertz, D.; Conen, A.; Heim, D.; Christen, S.; Gratwohl, A.; Battegay, M.; Widmer, A.F.; Fluckiger, U. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010, 45, 521–526.
- Jenks, J.D.; Cornely, O.A.; Chen, S.C.; Thompson, G.R., 3rd; Hoenigl, M. Breakthrough invasive fungal infections: Who is at risk? Mycoses 2020, 63, 1021–1032.
- Aldoss, I.; Dadwal, S.; Zhang, J.; Tegtmeier, B.; Mei, M.; Arslan, S.; Al Malki, M.M.; Salhotra, A.; Ali, H.; Aribi, A.; et al. Invasive fungal infections in acute myeloid leukemia treated with venetoclax and hypomethylating agents. Blood Adv. 2019, 3, 4043–4049.
- Infante, M.S.; Fernández-Cruz, A.; Núñez, L.; Carpio, C.; Jiménez-Ubieto, A.; López-Jiménez, J.; Vásquez, L.; Del Campo, R.; Romero, S.; Alonso, C.; et al. Grupo Español de Linfomas y Trasplante Autólogo de Medula Ósea (GELTAMO). Severe infections in patients with lymphoproliferative diseases treated with new targeted drugs: A multicentric real-world study. Cancer Med. 2021, 10, 7629–7640.
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for action: Invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin. Infect. Dis. 2018, 66, 140–148.
- Little, J.S.; Weiss, Z.F.; Hammond, S.P. Invasive Fungal Infections and Targeted Therapies in Hematological Malignancies. J. Fungi 2021, 7, 1058.
- Brown, J.R.; Byrd, J.C.; Coutre, S.E.; Benson, D.M.; Flinn, I.; Wagner-Johnston, N.D.; Spurgeon, S.E.; Kahl, B.S.; Bello, C.; Webb, H.K.; et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014, 123, 3390–3397.
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.; Cornely, O.A.; et al. European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230.
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509.
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516.
- Senjo, H.; Higuchi, T.; Okada, S.; Takahashi, O. Hyperferritinemia: Causes and significance in a general hospital. Hematology 2018, 23, 817–822.
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15.
- Gattermann, N. Iron overload in myelodysplastic syndromes (MDS). Int. J. Hematol. 2018, 107, 55–63.
- Shah, F.T.; Porter, J.B.; Sadasivam, N.; Kaya, B.; Moon, J.C.; Velangi, M.; Ako, E.; Pancham, S. Guidelines for the monitoring and management of iron overload in patients with haemoglobinopathies and rare anaemias. Br. J. Haematol. 2022, 196, 336–350.
- Oliva, E.N.; Huey, K.; Deshpande, S.; Turner, M.; Chitnis, M.; Schiller, E.; Tang, D.; Yucel, A.; Hughes, C.; Shah, F. A Systematic Literature Review of the Relationship between Serum Ferritin and Outcomes in Myelodysplastic Syndromes. J. Clin. Med. 2022, 11, 895.
- Malcovati, L.; Della Porta, M.G.; Cazzola, M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006, 91, 1588–1590.
- Weber, S.; Parmon, A.; Kurrle, N.; Schnütgen, F.; Serve, H. The Clinical Significance of Iron Overload and Iron Metabolism in Myelodysplastic Syndrome and Acute Myeloid Leukemia. Front. Immunol. 2021, 11, 627662.
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspect. Biol. 2013, 5, a012559.
- Park, S.; Sapena, R.; Kelaidi, C.; Vassilieff, D.; Bordessoule, D.; Stamatoullas, A.; Cheze, S.; Beyne-Rauzy, O.; Vey, N.; Rose, C.; et al. Ferritin level at diagnosis is not correlated with poorer survival in non RBC transfusion dependent lower risk de novo MDS. Leuk. Res. 2011, 35, 1530–1533.
- Waszczuk-Gajda, A.; Madry, K.; Machowicz, R.; Drozd-Sokołowska, J.; Stella-Hołowiecka, B.; Mital, A.; Obara, A.; Szmigielska-Kapłon, A.; Sikorska, A.; Subocz, E.; et al. Red blood cell transfusion dependency and hyperferritinemia are associated with impaired survival in patients diagnosed with myelodysplastic syndromes: Results from the first Polish MDS-PALG Registry. Adv. Clin. Exp. Med. 2016, 25, 633–641.
- Kuo, C.H.; Dai, Z.K.; Wu, J.R.; Hsieh, T.-J.; Hung, C.-H.; Hsu, J.-H. Septic arthritis as the initial manifestation of fatal Vibrio vulnificus septicemia in a patient with thalassemia and iron overload. Pediatric Blood Cancer 2009, 53, 1156–1158.
- Barton, J.C.; Acton, R.T. Hemochromatosis and Vibrio vulnificus wound infections. J. Clin. Gastroenterol. 2009, 43, 890–893.
- Bergmann, T.K.; Vinding, K.; Hey, H. Multiple hepatic abscesses due to Yersinia enterocolitica infection secondary to primary haemochromatosis. Scand. J. Gastroenterol. 2001, 36, 891–895.
- Portugal, S.; Carret, C.; Recker, M.; Armitage, A.; Gonçalves, L.A.; Epiphanio, S.; Sullivan, D.; Roy, C.; Newbold, C.; Drakesmith, A.; et al. Host-mediated regulation of superinfection in malaria. Nat. Med. 2011, 17, 732–737.
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 82, 29–40.
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 67, 133–143.
- Macdougall, I.C.; Bircher, A.J.; Eckardt, K.U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M.; et al. Iron management in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes’ (KDIGO) controversies conference. Kidney Int. 2016, 89, 28–39.
- Fernández-Ruiz, M.; Parra, P.; Ruiz-Merlo, T.; López-Medrano, F.; Juan, R.S.; Polanco, N.; González, E.; Andrés, A.; Aguado, J.M. Association between baseline serum hepcidin levels and infection in kidney transplant recipients: Potential role for iron overload. Transpl. Infect. Dis. 2018, 20, e12807.
- Chow, J.K.L.; Ganz, T.; Ruthazer, R.; Simpson, M.A.; Pomfret, E.A.; Gordon, F.D.; Westerman, M.E.; Snydman, D. Iron-related markers are associated with infection after liver transplantation. Liver Transpl. 2017, 23, 1541–1552.
- Tachibana, T.; Tanaka, M.; Takasaki, H.; Numata, A.; Ito, S.; Watanabe, R.; Hyo, R.; Ohshima, R.; Hagihara, M.; Sakai, R.; et al. Pretransplant serum ferritin levels are associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int. J. Hematol. 2011, 93, 368–374.
- Kanda, J.; Mizumoto, C.; Ichinohe, T.; Kawabata, H.; Saito, T.; Yamashita, K.; Kondo, T.; Takakura, S.; Ichiyama, S.; Uchiyama, T.; et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011, 46, 208–216.
- Miceli, M.H.; Dong, L.; Grazziutti, M.L.; Fassas, A.; Thertulien, R.; Van Rhee, F.; Barlogie, B.; Anaissie, E.J. Iron overload is a major risk factor for severe infection after autologous stem cell transplantation: A study of 367 myeloma patients. Bone Marrow Transplant. 2006, 37, 857–864.
- Valkovic, T.; Gacic, V.; Nacinovic-Duletic, A. Multiple Myeloma Index for Risk of Infections. J. Cancer 2018, 9, 2211–2214.
- Bairwa, G.; Hee Jung, W.; Kronstad, J.W. Iron acquisition in fungal pathogens of humans. Metallomics 2017, 9, 215–227.
- Kosman, D.J. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 2003, 47, 1185–1197.
- Morrissey, J.A.; Williams, P.H.; Cashmore, A.M. Candida albicans has a cell-associated ferric-reductase activity which is regulated in response to levels of iron and copper. Microbiology 1996, 142 Pt 3, 485–492.
- Knight, S.A.; Lesuisse, E.; Stearman, R.; Klausner, R.D.; Dancis, A. Reductive iron uptake by Candida albicans: Role of copper, iron and the TUP1 regulator. Microbiology 2002, 148, 29–40.
- Knight, S.A.; Vilaire, G.; Lesuisse, E.; Dancis, A. Iron acquisition from transferrin by Candida albicans depends on the reductive pathway. Infect. Immun. 2005, 73, 5482–5492.
- Ramanan, N.; Wang, Y. A high-affinity iron permease essential for Candida albicans virulence. Science 2000, 288, 1062–1064.
- Jeeves, R.E.; Mason, R.P.; Woodacre, A.; Cashmore, A.M. Ferric reductase genes involved in high-affinity iron uptake are differentially regulated in yeast and hyphae of Candida albicans. Yeast 2011, 28, 629–644.
- Blatzer, M.; Binder, U.; Haas, H. The metalloreductase FreB is involved in adaptation of Aspergillus fumigatus to iron starvation. Fungal Genet. Biol. 2011, 48, 1027–1033.
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237.
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451.
- Winkelmann, G. Ecology of siderophores with special reference to the fungi. Biometals 2007, 20, 379–392.
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187.
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 2004, 200, 1213–1219.
- Hissen, A.H.; Wan, A.N.; Warwas, M.L.; Pinto, L.J.; Moore, M.M. The Aspergillus fumigatus siderophore bio-synthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 2005, 73, 5493–5503.
- Howard, D.H.; Rafie, R.; Tiwari, A.; Faull, K.F. Hydroxamate siderophores of Histoplasma capsulatum. Infect. Immun. 2000, 68, 2338–2343.
- Schrettl, M.; Haas, H. Iron homeostasis–Achilles’ heel of Aspergillus fumigatus? Curr. Opin. Microbiol. 2011, 14, 400–405.
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 2003, 62, 316–330.
- Philpott, C.C. Iron uptake in fungi: A system for every source. Biochim. Biophys. Acta 2006, 1763, 636–645.
- Lesuisse, E.; Blaiseau, P.L.; Dancis, A.; Camadro, J.M. Siderophore uptake and use by the yeast Saccharomyces Cerevisiae. Microbiology 2001, 147, 289–298.
- Pao, S.S.; Paulsen, I.T.; Saier, M.H. Major, Facilitator Superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34.
- Philpott, C.C.; Protchenko, O. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot. Cell 2008, 7, 20–27.
- Yun, C.W.; Tiedeman, J.S.; Moore, R.E.; Philpott, C.C. Siderophore-iron uptake in Saccharomyces cerevisiae. Identification of ferrichrome and fusarinine transporters. J. Biol. Chem. 2000, 275, 16354–16359.
- Heymann, P.; Gerads, M.; Schaller, M.; Dromer, F.; Winkelmann, G.; Ernst, J.F. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 2002, 70, 5246–5255.
