Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
|
Materials Science, Coatings & Films
|
Materials Science, Characterization & Testing
Natural biopolymers, especially carbohydrates, show excellent material properties, such as mechanical strength, plasticity, and biodegradability. In addition, the anionic charges of hydrophilic polysaccharides (such as sulfate polysaccharides, hyaluronic acid, etc.) allow ionic attraction with metal ions or organic salts, and thereby they achieve antibacterial material properties. These antibacterial materials can be used to make implants for biomedical use. However, in-vivo compatibility remains a major limitation of such polymeric materials.
- Natural Polysaccharides
- Chitosan
- Sulfated Polysaccharides
- Hyaluronic Acid
- κ-Carrageenan
- Pectins
- Dextran
1. Composition of Biofilms: Syntropy of Bacteria
Biofilms comprise extracellular polymeric substances (abbreviated as EPS), mainly polysaccharides. In Gram-negative bacteria, some polysaccharides could be neutral or polyanionic. However, the polyanionic nature of these polysaccharides is characterized primarily by uronic acids (such as D-glucuronic, D-galacturonic, and mannuronic acids) or ketal-linked pyruvate and, therefore, imparts a negative charge over the microbial biofilm surfaces [1]. This negative charge over the microbial biofilm surface helps in the association of these biofilm layers with divalent metal cations such as Ca2+ and Mg2+, which further enhances cross-linking among these polymers and the development of biofilm. On the contrary, in some Gram-positive bacteria, such as the staphylococci, the chemical composition of EPS could be different due to their cationic nature. Apart from polysaccharides, biofilm contains lipids, proteins, nucleic acids, and humic substances. EPS could be hydrophobic or amphiphilic. However, the presence of a large number of hydroxyl groups of polysaccharides in EPS incorporates water molecules via hydrogen bonding; therefore, the composition and structure of these polysaccharides play an essential role in determining their primary conformation and physicochemical functions of the biofilm [2], e.g., bacterial EPS, which contains a backbone of 1,3- or 1,4-β-linked hexose residues, characterizes low solubility.
Interestingly, EPS is not uniformly distributed and is also attributed by the age of biofilm and concentration of metal ions and macromolecules, and even its biosynthesis can severely be limited or enhanced by levels of carbon, nitrogen, potassium, and phosphate [1]. The dynamicity of composition in biofilm architecture is continuously affected by internal and external processes. As microcolonies of bacteria live in syntropy, they continuously exchange their genetic material or antibiotic-resistant gene, quorum sensing, and cycling of nutrients.
In conclusion, bacteria of biofilms are difficult to target through conventional antibiotics. Secondly, secondary infections are prominently influenced by these bacteria of biofilms; therefore, much interest has been drawn towards the exploration of polymeric materials that can be used to make commercial biomedical products. While several examples of polymeric materials have been cited in the literature, their lower availability, higher cost, and biodegradability has restricted their direct use to becoming a preferred choice of interest.
2. Natural Polymeric Carbohydrate-Based Antibiofilm Materials
2.1. Sulfated Polysaccharides
A sulfated polysaccharide (fucoidan) isolated from marine algae (Fucus vesiculosus) showed activity against biofilm-forming bacteria [3][4], as shown in Figure 4A. The minimum inhibitory concentrations against food-borne bacteria (Listeria monocytogenes = 250 μg mL−1; Staphylococcus aureus = 500 μg mL−1) and dental plaque bacteria (Enterococcus faecalis = 1000 μg mL−1, Streptococcus mutans = 125 μg mL−1; Streptococcus oralis = 500 μg mL−1; Streptococcus sobrinus = 250 μg mL−1) have been recorded [3]. Figure 4B shows the structure of dermatan sulfate (glycosaminoglycan), and its co-immobilization with chitosan on polyethylene terephthalate surfaces prevents Staphylococcus epidermidis from forming a biofilm.
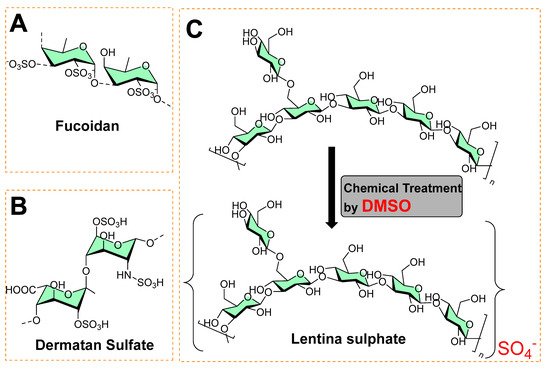
Figure 4. (A) Chemical structure of fucoidan. (B) Chemical structure of dermatan sulfate. (C) Chemical synthesis of lentina sulfate using DMSO.
In another study, Lentina, a mushroom polysaccharide, derivatized with the help of dimethyl sulfoxide into sulfate form [5], is shown in Figure 4C. This chemical transformation enhanced the water-solubility of the sulfated form as anionic polymers, which made it possible to combine with polycationic chitosan deposited onto the surfaces of polyurethane (PU) via the layer-by-layer (LbL) assembly technique [5]. These polymeric coatings showed a significant inhibition of the growth of Pseudomonas aeruginosa and reduced fibrinogen adsorption and platelet adhesion [5]. In other study, an extract containing sulfated polysaccharides (SPs) from green algae (Chlamydomonas reinhardtii) was studied for antibacterial and antibiofilm activity at 0.5 mg mL−1. It exhibited 34.52, 48.6, 66.1, and 55.6% reduced colony-forming units (CFU) against Bacillus subtilis, Streptococcus, Neisseria mucosa, and Escherichia coli, respectively [6].
2.2. Hyaluronic Acid
A grafted copolymer derivative (HA-EDA-BMP-MANa) of hyaluronic acid (HA) with ethylamine (EDA), and a methyl propionic acid (BMP) polymethacrylate structure (MANa, as shown in Figure 5) was successfully reported using atom transfer radical polymerization (ATRP chemistry) [7][8], as shown in Figure 5. The presence of ionic carboxylic and amino groups in the prepared copolymer (HA-EDA-BMP-MANa) directed its formulation (at three different pH values, namely, 5, 6, and 7) into a hydrogel at a concentration of 10% w/v, with or without vancomycin (2% w/v). In addition, a sustain pH-dependent in vitro vancomycin release over 48 h was observed, exhibiting biomaterial improved properties against Staphylococcus aureus adhesion on titanium disks compared to the other reported unmodified hyaluronic acid hydrogel [9].
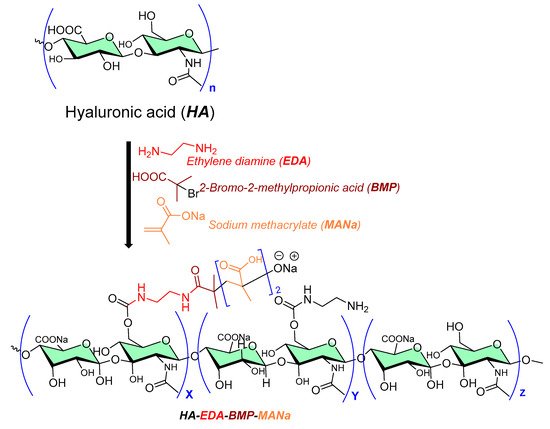
Figure 5. Chemical processing of hyaluronic acid copolymer as antibiofilm surface material.
2.3. Pectins
Pectins are polysaccharides consisting primarily of esterified D-galacturonic acid that resides in an α-(1–4) chain, as shown in Figure 6. Alkaline processes hydrolyze ester groups, exposing more carboxylic groups for ionic interaction with metals (silver nanoparticles [10][11]) or organic salts (benzalkonium chloride [12], dodecyl trimethylammonium chloride [13]) and imparts antibiofilm activity.
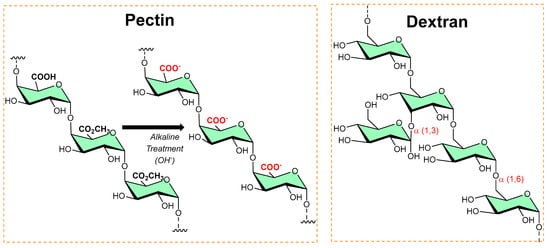
Figure 6. The typical chemical transformation of pectin into a more functional form, while the dextran structure shows alpha linkages and available functionalized sites.
2.4. Dextran
Dextran is a long-chain glucose homopolysaccharide with primarily α-1,6-glucopyranosidic linkages (shown in Figure 6) and is produced from sucrose by Leuconostoc mesenteroides and related bacteria, and from dextrin by other bacteria. Its candidature as an implant material, dextran-70 (mol. mass ≈ 70 000), can be retained in the intravascular spaces and contribute to the colloid plasma oncotic pressure. However, in high concentrations, dextran-70 inhibits the aggregation of platelets and enables fibrinolysis. In addition, dextran–chitosan gel showed antibiofilm activity [14]; Li et al. reported an acid-induced self-catalyzing material (dextran-coated copper peroxide nanoaggregates (DCPNAs) for antibiofilm [15]; Hoque et al. reported sustained-release antibacterial dextran hydrogels to eradicate the microbial biofilms [16]; Naha et al. reported dextran-coated iron oxide nanoparticles for pH-based biofilm disruption [17].
2.5. κ-Carrageenan
Structurally, κ-carrageenan is a linear sulfated polysaccharide (shown in Figure 7) commonly found in red seaweed. Green microwave synthesis was used to form κ-carrageenan–silver nanoparticle (CRG–Ag) nanocomposites of size 50 ± 10 nm, and they were found to be active against Staphylococcus aureus- and Pseudomonas aeruginosa-mediated biofilms [18][30]. Additionally, CRG–Ag nanoparticles encapsulated in potassium chloride cross-linked hydrogel displayed reasonable thermal stability and antimicrobial activity [19].
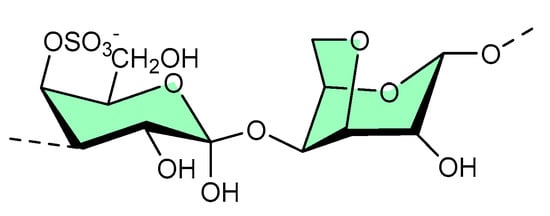
Figure 7. Available site for material functionalization on κ-carrageenan structure.
3. Chitosan as Polymeric Material Surface
Chitosan is the second most abundant polysaccharide in nature. Most of its natural proportion is found in the form of the exoskeletal coat of arthropods [20][21]. Its remarkable physicochemical properties, such as biocompatibility, biodegradability, and low toxicity, make chitosan a suitable material for various biomedical uses [21][22]. It is an amino polysaccharide linear polycation biopolymer (as shown in Figure 8), randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) with a high charge density, reactive hydroxyl and amino groups, along with extensive hydrogen bonding, which enhances its physical stability and processability [23]. However, despite its characteristics, it exhibits poor solubility due to extensive hydrogen bonds and acetamido groups in the crystalline state. The presence of polycationic charges on chitosan imparts its antimicrobial properties. These positively charged amino groups of glucosamine of chitosan interact with negatively charged membrane constituents of microbes, inducing leakage of intracellular materials of the cell and disrupting the membrane function, as found in Gram-negative bacteria (including Escherichia coli and Salmonella typhimurium), Gram-positive bacteria (Staphylococcus aureus) [24], and yeast (Saccharomyces cerevisiae) [25][26][27].
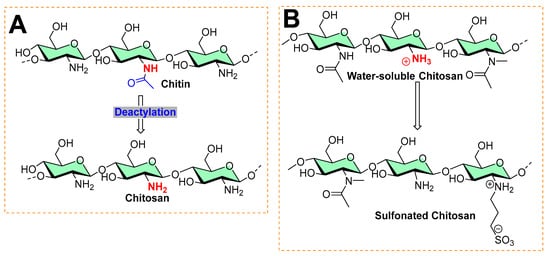
Figure 8. Typical processes in chitosan chemistry: (A) commercial preparation from chitin by deacetylation is found in the exoskeleton of crustaceans and the cell wall of fungi. (B) Sulfonation of chitosan.
Different Forms of Chitosan and its Quaternization as Antibiofilm Activity
Various forms of chitosan have shown antibiofilm activity [26]. Factors such as the molecular weight (high molecular weight HMW, medium molecular weight MMW, low molecular weight LMW), degree of deacetylation/acetylation, and derivatization affect the antibiofilm activity of chitosan [26]. Both versions of chitosan—HMW (624 KDa) with a degree of deacetylation >75%, and LMW (107 KDa) with a degree of deacetylation of 75–85%—inhibit the adhesion and maturity of S. mutants [27] and prevent the growth of planktonic cells and adhesion of vancomycin-resistant Staphylococcus aureus and Enterococcus faecalis [28]. Furthermore, LMW (107 KDa, with a degree of deacetylation of 75–85%) chitosan prevented the growth, adhesion, and biofilm formation of methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible Staphylococcus aureus (MSSA), and methicillin-resistant Staphylococcus epidermidis (MRSE). These studies verify the intrinsic antibiofilm potency of chitosan [26].
To enhance the antibiofilm activity of chitosan, researchers attempted various derivatizations: (a) carboxymethyl chitosan (30 KDa) with a degree of deacetylation (90%) inhibited the broad spectrum antibiofilm activity of Lactobacillus gasseri, Streptococcus salivarius, Rothia dentocariosa, and Staphylococcus epidermidis [29]; 2-methylaziridine-modified chitooligosaccharide changed the fluidity of biofilm and the bacterial cell membrane of Pseudomonas aeruginosa by releasing nitric oxide [30]. Among derivations, quaternization is a common strategy as it provides an ionic charge that can be used for adsorption of the oppositely charged ions/functional molecules. For example, hydroxypropyl-trimethyl ammonium chloride chitosan (HACC), with a degree of deacetylation of 91.83%, prevented biofilm growth of Staphylococcus aureus and Staphylococcus epidermidis by targeting the extracellular polysaccharides-encoded gene (icaA) expression [31]; (b) N,N,N-trimethylchitosans showed broad-spectrum antibiofilm activity against Gram-positive (Staphylococcus epidermidis) and Gram-negative bacteria (Escherichia coli) [32]; (c) Quaternary ammoniumyl chitosan (degree of deacetylation = 96%) derivatives prevented the biofilms of Staphylococcus aureus [33] and other examples, as reported in the following reports [34][35].
This entry is adapted from the peer-reviewed paper 10.3390/mi13081265
References
- Ian W. Sutherland; Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 2001, 147, 3-9, 10.1099/00221287-147-1-3.
- Rodney M. Donlan; Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases 2002, 8, 881-890, 10.3201/eid0809.020063.
- Joon-Young Jun; Min-Jeong Jung; In-Hak Jeong; Koji Yamazaki; Yuji Kawai; Byoung-Mok Kim; Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Marine Drugs 2018, 16, 301, 10.3390/md16090301.
- Ratih Pangestuti; Se-Kwon Kim; Neuroprotective Effects of Marine Algae. Marine Drugs 2011, 9, 803-818, 10.3390/md9050803.
- Yifeng Wang; Qunfeng Hong; Yanjun Chen; Xinxin Lian; Yanfei Xiong; Surface properties of polyurethanes modified by bioactive polysaccharide-based polyelectrolyte multilayers. Colloids and Surfaces B: Biointerfaces 2012, 100, 77-83, 10.1016/j.colsurfb.2012.05.030.
- J. Vishwakarma; S.L. Vavilala; Evaluating the antibacterial and antibiofilm potential of sulphated polysaccharides extracted from green algaeChlamydomonas reinhardtii. Journal of Applied Microbiology 2019, 127, 1004-1017, 10.1111/jam.14364.
- Calogero Fiorica; Giovanna Pitarresi; Fabio Salvatore Palumbo; Mauro Di Stefano; Filippo Calascibetta; Gaetano Giammona; A new hyaluronic acid pH sensitive derivative obtained by ATRP for potential oral administration of proteins. International Journal of Pharmaceutics 2013, 457, 150-157, 10.1016/j.ijpharm.2013.09.005.
- Fabio Salvatore Palumbo; Giovanna Pitarresi; Calogero Fiorica; Pietro Matricardi; Antonella Albanese; Gaetano Giammona; In situ forming hydrogels of new amino hyaluronic acid/benzoyl-cysteine derivatives as potential scaffolds for cartilage regeneration. Soft Matter 2012, 8, 4918-4927, 10.1039/c2sm07310b.
- Fabio Salvatore Palumbo; Antonella Bavuso Volpe; Maria Grazia Cusimano; Giovanna Pitarresi; Gaetano Giammona; Domenico Schillaci; A polycarboxylic/amino functionalized hyaluronic acid derivative for the production of pH sensible hydrogels in the prevention of bacterial adhesion on biomedical surfaces. International Journal of Pharmaceutics 2015, 478, 70-77, 10.1016/j.ijpharm.2014.11.015.
- P. Pallavicini; Carla Renata Arciola; Federico Bertoglio; S. Curtosi; Giacomo Dacarro; A. D'Agostino; F. Ferrari; D. Merli; C. Milanese; S. Rossi; et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. Journal of Colloid and Interface Science 2017, 498, 271-281, 10.1016/j.jcis.2017.03.062.
- Federico Bertoglio; Lorenzo De Vita; Agnese D’Agostino; Yuri Diaz Fernandez; Andrea Falqui; Alberto Casu; Daniele Merli; Chiara Milanese; Silvia Rossi; Angelo Taglietti; et al. Increased Antibacterial and Antibiofilm Properties of Silver Nanoparticles Using Silver Fluoride as Precursor. Molecules 2020, 25, 3494, 10.3390/molecules25153494.
- S. Khelissa; A. Gharsallaoui; A. Fadel; A. Barras; C. Jama; F. Jbilou; N.‐E. Chihib; Microencapsulation of benzalkonium chloride enhanced its antibacterial and antibiofilm activities against Listeria monocytogenes and Escherichia coli. Journal of Applied Microbiology 2021, 131, 1136-1146, 10.1111/jam.15010.
- Simon Khelissa; Adem Gharsallaoui; Jian Wang; Emilie Dumas; Alexandre Barras; Charafeddine Jama; Fouzia Jbilou; Noureddine Loukili; Nour-Eddine Chihib; Anti-biofilm activity of dodecyltrimethylammonium chloride microcapsules against Salmonella enterica serovar Enteritidis and Staphylococcus aureus. Biofouling 2021, 37, 49-60, 10.1080/08927014.2021.1873958.
- Sathish Paramasivan; Damien Jones; Leonie Baker; Lyall Hanton; Simon Robinson; Peter J. Wormald; Lorwai Tan; The Use of Chitosan–Dextran Gel Shows Anti-Inflammatory, Antibiofilm, and Antiproliferative Properties in Fibroblast Cell Culture. American Journal of Rhinology & Allergy 2014, 28, 361-365, 10.2500/ajra.2014.28.4069.
- Min Li; Xi Lan; Ximei Han; Shuo Shi; Hao Sun; Yi Kang; Jie Dan; Jing Sun; Wentao Zhang; Jianlong Wang; et al. Acid-Induced Self-Catalyzing Platform Based on Dextran-Coated Copper Peroxide Nanoaggregates for Biofilm Treatment. ACS Applied Materials & Interfaces 2021, 13, 29269-29280, 10.1021/acsami.1c03409.
- Jiaul Hoque; Jayanta Haldar; Direct Synthesis of Dextran-Based Antibacterial Hydrogels for Extended Release of Biocides and Eradication of Topical Biofilms. ACS Applied Materials & Interfaces 2017, 9, 15975-15985, 10.1021/acsami.7b03208.
- Pratap C. Naha; Yuan Liu; Geelsu Hwang; Yue Huang; Sarah Gubara; Venkata Jonnakuti; Aurea Simon-Soro; Dongyeop Kim; Lizeng Gao; Hyun Koo; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960-4971, 10.1021/acsnano.8b08702.
- Apoorva Goel; Mukesh Kumar Meher; Payal Gupta; Khushboo Gulati; Vikas Pruthi; Krishna Mohan Poluri; Microwave assisted κ-carrageenan capped silver nanocomposites for eradication of bacterial biofilms. Carbohydrate Polymers 2018, 206, 854-862, 10.1016/j.carbpol.2018.11.033.
- Apoorva Goel; Mukesh Kumar Meher; Payal Gupta; Khushboo Gulati; Vikas Pruthi; Krishna Mohan Poluri; Microwave assisted κ-carrageenan capped silver nanocomposites for eradication of bacterial biofilms. Carbohydrate Polymers 2018, 206, 854-862, 10.1016/j.carbpol.2018.11.033.
- Daniel Elieh-Ali-Komi; Michael R Hamblin; Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials.. International Journal of Advanced Research 2016, 4, 411-427, .
- Ahmad Sukari Halim; Mercy Halleluyah Periayah; Arman Zaharil Mat Saad; Chitosan: A promising marine polysaccharide for biomedical research. Pharmacognosy Reviews 2016, 10, 39-42, 10.4103/0973-7847.176545.
- Dina Raafat; Hans-Georg Sahl; Chitosan and its antimicrobial potential - a critical literature survey. Microbial Biotechnology 2009, 2, 186-201, 10.1111/j.1751-7915.2008.00080.x.
- Jae-Young Je; Se-Kwon Kim; Chitosan Derivatives Killed Bacteria by Disrupting the Outer and Inner Membrane. Journal of Agricultural and Food Chemistry 2006, 54, 6629-6633, 10.1021/jf061310p.
- Anna Zakrzewska; Andre Boorsma; Stanley Brul; Klaas J. Hellingwerf; Frans M. Klis; Transcriptional Response of Saccharomyces cerevisiae to the Plasma Membrane-Perturbing Compound Chitosan. Eukaryotic Cell 2005, 4, 703-715, 10.1128/ec.4.4.703-715.2005.
- I.M Helander; E.-L Nurmiaho-Lassila; R Ahvenainen; J Rhoades; S Roller; Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. International Journal of Food Microbiology 2001, 71, 235-244, 10.1016/s0168-1605(01)00609-2.
- Fazlurrahman Khan; Dung Thuy Nguyen Pham; Sandra Folarin Oloketuyi; Panchanathan Manivasagan; Junghwan Oh; Young-Mog Kim; Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids and Surfaces B: Biointerfaces 2019, 185, 110627, 10.1016/j.colsurfb.2019.110627.
- Eduardo Costa; S. Silva; Freni Tavaria; M.M. Pintado; Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe 2013, 20, 27-31, 10.1016/j.anaerobe.2013.02.002.
- Eduardo Costa; Sara Silva; Mariana Veiga; Sandra Vicente; Freni K. Tavaria; Manuela E. Pintado; Investigation of chitosan’s antibacterial activity against vancomycin resistant microorganisms and their biofilms. Carbohydrate Polymers 2017, 174, 369-376, 10.1016/j.carbpol.2017.06.087.
- Yulong Tan; Matthias Leonhard; Doris Moser; Su Ma; Berit Schneider-Stickler; Inhibition of mixed fungal and bacterial biofilms on silicone by carboxymethyl chitosan. Colloids and Surfaces B: Biointerfaces 2016, 148, 193-199, 10.1016/j.colsurfb.2016.08.061.
- David B. Hill; Katelyn P. Reighard; Graham A. Dixon; Brittany V. Worley; Mark H. Schoenfisch; Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. null 1970, 31, 775–787, 10.6084/m9.figshare.1613321.
- Zhao-Xiang Peng; Bing Tu; Yang Shen; Lin Du; Ling Wang; Sheng-Rong Guo; Ting-Ting Tang; Quaternized Chitosan Inhibits icaA Transcription and Biofilm Formation by Staphylococcus on a Titanium Surface. Antimicrobial Agents and Chemotherapy 2011, 55, 860-866, 10.1128/aac.01005-10.
- Fuguang Jiang; Ying Deng; Chih-Ko Yeh; Yuyu Sun; Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. Journal of Materials Chemistry B 2014, 2, 8518-8527, 10.1039/c4tb01131g.
- Priyanka Sahariah; Már Másson; Rikke Louise Meyer; Quaternary Ammoniumyl Chitosan Derivatives for Eradication of Staphylococcus aureus Biofilms. Biomacromolecules 2018, 19, 3649-3658, 10.1021/acs.biomac.8b00718.
- Yukun Qin; Pengcheng Li; Zhanyong Guo; Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydrate Polymers 2020, 236, 116002, 10.1016/j.carbpol.2020.116002.
- Ross P. Carlson; Reed Taffs; William M. Davison; Philip S. Stewart; Anti-biofilm properties of chitosan-coated surfaces. Journal of Biomaterials Science, Polymer Edition 2008, 19, 1035-1046, 10.1163/156856208784909372.
- Ross P. Carlson; Reed Taffs; William M. Davison; Philip S. Stewart; Anti-biofilm properties of chitosan-coated surfaces. Journal of Biomaterials Science, Polymer Edition 2008, 19, 1035-1046, 10.1163/156856208784909372.
This entry is offline, you can click here to edit this entry!
