Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Carbamates are widely used and known around the world as pesticides in spite of also having medical applications. Carbamates are mostly used as pesticides worldwide, despite their interesting medical applications, such as in the treatment of myasthenia gravis, Alzheimer’s disease, or glaucoma, among others
- carbamates
- male fertility
- endocrine disruptors
- endocrine system
1. Introduction
Carbamates are mostly used as pesticides worldwide, despite their interesting medical applications, such as in the treatment of myasthenia gravis, Alzheimer’s disease, or glaucoma, among others [1]. Carbamates were firstly extracted from a West African plant, Calabar bean, Physostigma venenosum, and are classified as esters of N-methyl carbamic acid [2]. This class of chemicals is known to lack residue persistency in the environment and in mammalian species due to their rapid hydrolyzation to, usually, less toxic metabolites, amine, and carbon dioxide (CO2), which are easily excreted from the organism [3]. However, because of their lack of species selectivity, and their lipophilicity and toxicity, its overexposure and/or chronic exposure poses a serious threat to the environment, to human beings and to other animal species [4][5]. Compared to other classes of pesticides, carbamates exhibit one of the widest ranges of subtypes (carbendazim, carbaryl, carbofuran, aminocarb, thiodicarb, mancozeb, among others). Each compound has its application (e.g., insecticide, fungicide, and herbicide), characteristics, and harmful effects (as reviewed by King and Aaron [1]). Despite these differences, the compounds from the carbamate´s family share the common trait of being both acetylcholinesterase (AChE) inhibitors, through its carbamylation, and endocrine-disrupting chemicals (EDCs), interfering with the hypothalamic–pituitary–testicular (HPT) axis, which may cause several reproductive problems [4][6][7][8].
The endocrine system consists of a series of glands throughout the body that produce and secrete hormones to regulate a wide range of physiological processes, such as respiration, metabolism, reproduction, sensory perception, movement, sexual development, and growth. The main hormone-producing glands are the hypothalamus, pituitary, parathyroid, pancreas, thyroid, adrenal, pineal, ovaries, and testis. Each gland produces and is under the influence of specific hormones or hormonal cues, controlling the body homeostasis. There are several factors that can influence the function of endocrine organs, including aging, certain diseases and conditions, stress, environment, and genetics (7). EDCs are compounds that can mimic the action of hormones, binding to their receptors and inhibiting or stimulating the respective cascade and the HPT axis. In addition, EDCs can also affect the concentration of hormones through the stimulation or inhibition of their degradation, availability, or synthesis [9].
2. The Role of Acetylcholine in Male Reproductive Function
The toxicity of carbamates occurs due the inhibition of the AChE through carbamylation, which stops the degradation of the neurotransmitter acetylcholine (ACh) and leads to its accumulation in brain synapses and neuromuscular junctions. At physiological conditions, synaptic AChE prevents the accumulation of ACh and consequent overstimulation of the ACh receptor through its hydrolyzation to acetic acid and choline. Next, acetic acid feeds into the Krebs cycle, while choline is taken up by the neuron and resynthesized into new ACh. In this way, AChE inhibition results in the accumulation of ACh, overstimulating the muscarinic and nicotinic ACh receptors (Figure 1). The accumulation of ACh affects the whole organism and the most observed symptoms include sweating, excessive salivation, constricted pupils, blurred vision, lacrimation, wheezing, gastrointestinal issues including nausea, vomiting, and diarrhea, urinary incontinence and, in more severe cases, paralysis, cyanosis, and respiratory issues, which can last from a few hours to a few days or weeks [1]. Contrarily to organophosphates, another class of anticholinesterase pesticides, carbamates reversibly inhibit AChE, and are, therefore, less toxic [4].
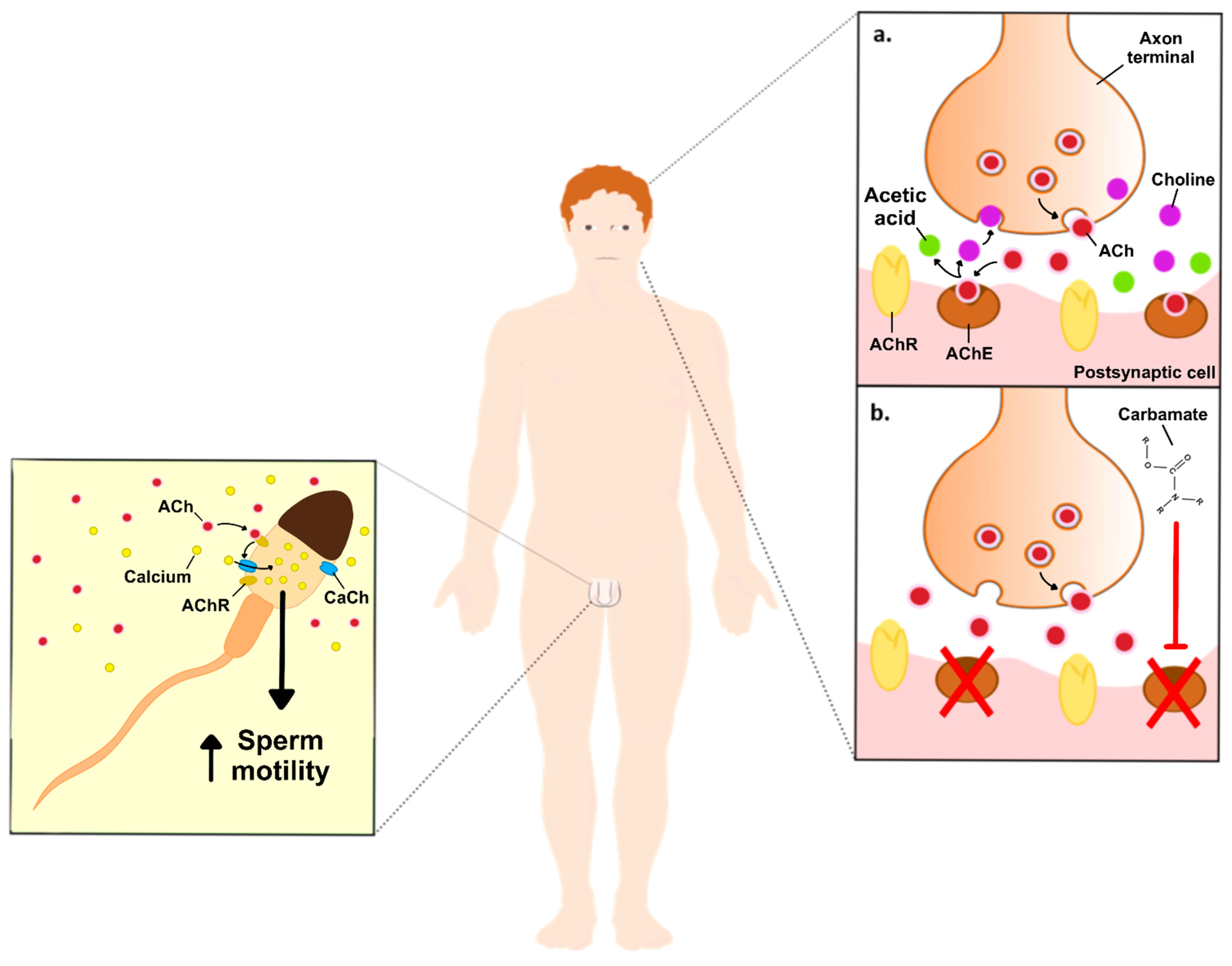
Figure 1. Schematic illustration of the effects of carbamates on neuronal ACh, and the hypothetical effects on non-neuronal ACh in the male reproductive system. Image right side: (a). ACh vesicles (red dots) are released to the synaptic clef, where they will bind to its receptors, while the excess is converted into acetic acid (green dots) and choline (purple dots), by the AChE. Choline is then taken up by the neuron to be resynthesized into new ACh. (b). Inhibition of AChE, upon exposure to carbamates, increases the levels of ACh on the synaptic cleft, thus producing its toxic effects. Image left side: ACh increases sperm motility through the stimulation of Ca2+ influx into the spermatozoa. Abbreviations: ACh—acetylcholine; AChE—acetylcholinesterase enzyme; AChR—acetylcholine receptor; CaCh-Ca2+ channels.
Biomarkers of toxicity of anti-AChE pesticides, like carbamates, were found to reduce epididymal and testicular sperm counts, as well as decrease serum testosterone concentration [10]. Many of the actions of ACh, including on the male reproductive system, are induced by its effects on muscarinic ACh receptors (mAChR) or nicotinic ACh receptors (nAChRs). The binding of acetylcholine to its receptors promotes Ca2+ influx, which activates Ca2+-dependent pathways including stimulation of the adenylyl cyclase activity and increased cyclic AMP (cAMP) levels, activation of the phosphoinositide 3-kinase (PI3K) pathway, and Ca2+/calmodulin-dependent protein kinase (CaMK)-dependent pathways [11][12]. The mAChR are located throughout the human organism, mainly at organs innervated by the parasympathetic system with particular emphasis on exocrine glands, including sweat and salivary glands, but also the heart, gastrointestinal tract, eye, and testis [13][14][15]. These include five distinct subtypes (M1-M5), and their activation is known to be involved in cell proliferation, differentiation, growth, among other functions. M1, M3 and M5 mAChRs preferentially couple to G-proteins of the Gq/11 family, while M2 and M4 mAChRs preferentially activate Gi/0-type G-proteins [16][17][18]. Han et al. studied the knockdown of M1, M3 and M5 and found that the M5 knockdown disrupted spermatogenesis and affected the expression of proteins in the blood-testis barrier (BTB) and ectoplasmic specializations. Furthermore, the M5 knockdown decreased the expression of pleckstrin homology-like domain, family B, member 2 (Phldb2), both in germ cells and Sertoli cells [15]. Phldb2 is a PH domain-containing protein that is highly sensitive to phosphatidylinositol 3,4,5-triphosphate (PIP3) and phosphatidylinositol 4,5-biphosphate (PIP2). Among its functions, Phldb2 has an important role in ACh receptor aggregation in the postsynaptic membrane, thus being a key component of synaptic podosomes, which are involved in the extracellular matrix remodeling [19]. Phldb2 also mediates focal adhesion disassembly as well as cell polarization and migration. Thus, the authors could conclude that the M5 knockdown decreased the expression of Phldb2 and disrupted the BTB and ectoplasmic specializations, impairing spermatogenesis [15].
Beyond mAChRs, ACh can also stimulate an unknown variety of ionotropic nAChRs with subtype-specific arrangements [20]. In more detail, these receptors belong to the super-family of homologous Cys-loop ion channel receptors, which consist of nine α and three β subunits. Both homomeric or heteromeric assembly of five subunits generate several distinct subtypes, which share a common basic structure but have specific pharmacological and functional properties [21]. Similar to mAChRs, the absence of nAChRs or the presence of reduced levels of ACh leads to reduced sperm motility, thus impairing male fertility [22]. Although the mechanisms remain to be elucidated, Bray et al. hypothesized that the nAChRs play a role on Ca2+ influx, which is essential for sperm motility and hyperactivation [22]. On the other hand, Arıcan et al. treated male Sprague–Dawley rats with acetamiprid (12.5, 25, and 35 mg/kg/day), a selective agonist of nAChRs, for 90 days and observed a decreased sperm count and increased apoptotic index in the testis upon exposure to the highest concentration. In addition, increased oxidative stress markers were reported, which were highlighted as the likely cause for the observed detrimental effects [23]. Taken together, these results suggest that nAChRs take part in a finely regulated pathway that modulates the testicular function, but further studies are needed to unveil the involved molecular mechanisms.
ACh was also found, along with crucial components of the cholinergic system, in mammalian cells not innervated by neurons, i.e., non-neuronal ACh and non-neuronal cholinergic system, respectively [24]. Namely, non-neuronal ACh was detected in the sperm of rabbit, bull, and man [25][26]. Moreover, there is evidence that the non-neuronal cholinergic system is widely expressed within the reproductive tract of male rats [27]. Via auto- and paracrine modes of action, and as previously mentioned for neuronal ACh, non-neuronal ACh also promotes cell proliferation and differentiation, as well as regulation of cell-cell contact, locomotion, and transport of ions and water [24][25][26][27][28]. Previous studies reported that interfering with the ACh cascade affects male reproductive potential. Sliwa observed that a high concentration of ACh (5 mg/mL) reduced in vitro mouse spermatozoa migration [29]. Sastry et al. reported that 1 μM of 2-benzoylethyltrimethylammonium (BETA), a choline acetyltransferase inhibitor, rapidly decreased human sperm motility (80% after 5 min treatment). In addition, a decrease in sperm motility by 95% was observed after 1 h treatment. Interestingly, these authors hypothesized that ACh is potentially synthesized by human sperm, exhibiting an autocrine and/or paracrine role [30]. Likewise, interferences in the non-neuronal cholinergic system caused by pharmacological substances like atropine, an anticholinergic drug that acts as a competitive yet reversible mAChR antagonist, were demonstrated to lead to impaired fertility, due to the inhibition of the contraction of the seminal vesicles during the process of emission. Sato et al. reported that male Sprague–Dawley rats treated with 125 mg/kg/day of atropine for 10–17 days had increased concentration of sperm in the vas deferens and observed a lower pregnancy rate when mated with females, as compared to the control group. These results led the authors to suggest that the inhibition of mAChRs by atropine led to the impairment of sperm and semen transport [31]. In a previous study, Ban et al. already had reported that Sprague–Dawley male rats treated with 125 mg/kg/day of atropine for 1 week led to a lower pregnancy rate. In addition, these authors also studied the effects of another mAChR antagonist, (2R)-N-[1-(6-aminopyridin-2-ylmethyl)piperidin-4-yl]-2-[(1R)-3,3-difluorocyclopentyl]-2-hydroxy-2-phenylacetamide, and reported that the treatment of 100 mg/kg/day for 4 weeks also lowered the pregnancy rates. Interestingly, no detrimental effects were observed after a 1-week withdrawal, suggesting that the effects of mAChR inhibition conferred by this compound are reversible [32]. More recently, it was reported in rats that the contraction of the testicular capsule, a complex fibrous structure consisting of various and distinct layers of tissues, like tunica albuginea, is associated with M3 receptors. Upon induction with cholinergic agonists, the activation of M3 receptors leads to Ca2+ influx through L-subtype and Ni2+ sensitive voltage-dependent Ca2+ channels, Ca2+ release from sarcoplasmic reticulum, as well as regulation of Ca2+ levels by the mitochondria. As such, and as explained by the authors, the contraction of testicular capsule has a functional involvement in sperm transport from the testes to epididymis. In this way, ACh present in non-neuronal sites may induce or modulate their contractions, allowing the correct movement of sperm from the testes [33]. In accordance, Nieto-Cerón et al. showed that AChE may play an important role in the male reproductive physiology, as they found significant levels in the human prostate [34]. Nevertheless, it remains to be studied whether the reduced cholinesterases (ChE) activities observed in semen are a cause or consequence of infertility, as the authors proposed [34]. Taken together, it is possible to conclude that ACh plays a regulatory role on male fertility, especially on spermatozoa, but further studies are needed to disclose the involved molecular mechanisms.
Carbamates can interfere within the normal neuronal ACh cascade, which means that, theoretically, they will also play a role in the regulation of non-neuronal ACh levels in the male reproductive system, since the non-neuronal cholinergic system is part of it. The direct effects of carbamates exposure to the testicular function, spermatogenesis, and sperm function, however, remains to be disclosed. Radhakrishnan et al. reported that elevated concentrations of ACh induced oxidative stress in the testis, thus diminishing sperm motility [35]. This outcome was found, however, to be reverted by a mild intramuscular dose of Botulinum toxin in the inferior spermatic nerve plexus, a place known to regulate steroidogenesis [35]. The Botulinum toxin acts through the cleavage of the SNARE proteins, preventing the release of ACh vesicles [36]. These data suggest that the carbamates-induced accumulation of ACh could lead to similar results, although further studies are needed to confirm this hypothesis.
3. Carbamates as Endocrine-Disrupting Chemicals (EDCs)
Carbamate pesticides have endocrine-disrupting properties that include disruption of the male reproductive system through the perturbation of the HPT axis, although the molecular mechanisms are not fully understood [4]. As the name indicates, this axis is composed by the hypothalamus, pituitary gland, and testes, and its normal function is crucial to provide the right amounts of hormones for male sexual development and function, and so any abnormality can lead to male infertility [37].
The hypothalamus is responsible for the secretion of pulsatile gonadotropin-releasing hormone (GnRH), which stimulates the synthesis and secretion of the gonadotropins luteinizing (LH) and follicle-stimulating (FSH), by the adenohypophysis. Then, FSH travels through the bloodstream and binds to its receptors on Sertoli cells, while the LH binds to the LH/chorionic gonadotropin receptor (LH/CG-R) present on the membrane of Leydig cells and stimulates steroidogenesis (Figure 2) [38][39]. The act of mobilizing cholesterol into mitochondria is the first and limiting step in the multi-phase process of testosterone production [40]. Cholesterol mobilization is mediated by the action of the translocator protein (TSPO) and the steroidogenic acute regulatory protein (StAR), both dependent on LH-induced increase on cAMP levels and cAMP-dependent phosphorylation by protein kinase A (PKA). Then, the cytochrome P450 enzyme (CYP11A1), which is present on the matrix side of the inner mitochondrial membrane, catalyzes the conversion of cholesterol into pregnenolone. Three enzymes—3β-hydroxysteroid dehydrogenase (HSD3B), 17α-hydroxylase/17,20 lyase (CYP17A1), and type 3 17β-hydroxysteroid dehydrogenase (HSD17B)—progressively convert pregnenolone into testosterone in the smooth endoplasmic reticulum [41]. Testosterone, then, regulates the pituitary’s production and secretion of LH through a negative feedback mechanism [42].
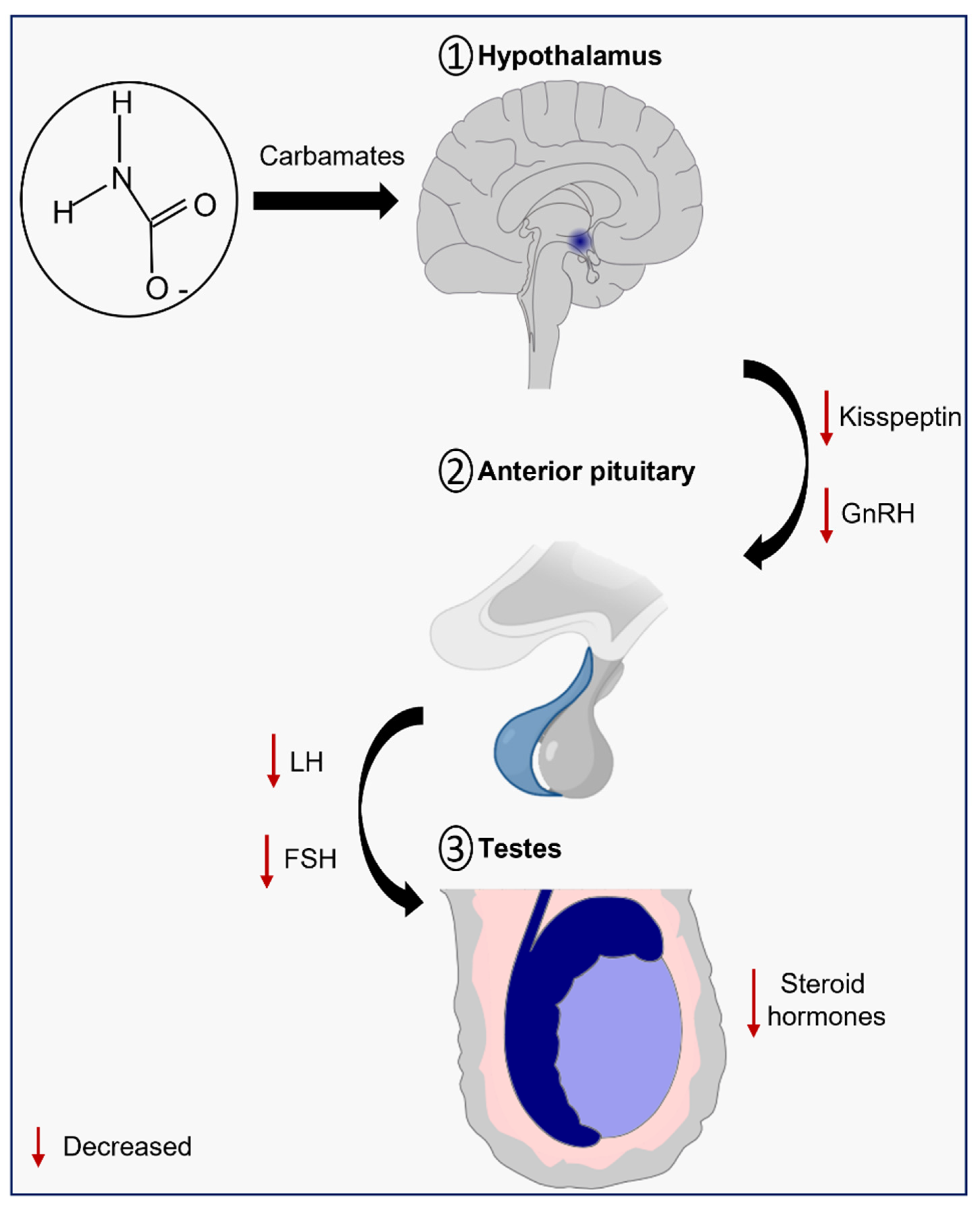
Figure 2. Schematic illustration of the effects of carbamates on the hypothalamic-pituitary-testicular (HPT) axis.
Several studies highlight the effects of carbamate exposure on the HPT axis. Taken together, it is suggested that exposure to carbamates impairs steroidogenesis through the downregulation of steroidogenic enzymes and/or toxicity to Leydig cells. The molecular mechanisms, however, are still poorly understood, which highlights the importance for further studies in this field.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23158206
References
- King, A.M.; Aaron, C.K. Organophosphate and carbamate poisoning. Emerg. Med. Clin. N. Am. 2015, 33, 133–151.
- Martin-Reina, J.; Duarte, J.A.; Cerrillos, L.; Bautista, J.D.; Moreno, I. Insecticide Reproductive Toxicity Profile: Organophosphate, Carbamate and Pyrethroids. J. Toxins 2017, 4, 1–7.
- WHO. Carbamate Pesticides: A General Introduction; World Health Organization: Geneva, Switzerland, 1986.
- Gupta, R.C.; Mukherjee, I.R.; Doss, R.B.; Malik, J.K.; Milatovic, D. Organophosphates and Carbamates. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 609–631.
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940.
- Afzal, S.; Shah, S.S.; Iqbal, M.F.; Noureen, A. Review on effect of carbamate pesticide on male reproductive system of mammals. Int. J. Entomol. Res. 2018, 3, 31–33.
- Liu, J.; Zhang, P.; Zhao, Y.; Zhang, H. Low dose carbendazim disrupts mouse spermatogenesis might Be through estrogen receptor related histone and DNA methylation. Ecotoxicol. Environ. Saf. 2019, 176, 242–249.
- Li, H.; Zhang, P.; Zhao, Y.; Zhang, H. Low doses of carbendazim and chlorothalonil synergized to impair mouse spermatogenesis through epigenetic pathways. Ecotoxicol. Environ. Saf. 2020, 188, 109908.
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The case of pesticides. Comptes Rendus Biol. 2017, 340, 406–409.
- Mor, I.; Soreq, H. Cholinergic Toxicity and the Male Reproductive System. Reprod. Dev. Toxicol. 2011, 66, 863–870.
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004, 25, 317–324.
- Harvey, R.D. Muscarinic receptor agonists and antagonists: Effects on cardiovascular function. Muscarinic Recept. 2012, 208, 299–316.
- Abrams, P.; Andersson, K.E.; Buccafusco, J.J.; Chapple, C.; De Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 148, 565–578.
- Borges, M.O.; Abreu, M.L.; Porto, C.S.; Avellar, M.C.W. Characterization of muscarinic acetylcholine receptor in rat Sertoli cells. Endocrinology 2001, 142, 4701–4710.
- Han, X.; Zhang, C.; Ma, X.; Yan, X.; Xiong, B.; Shen, W.; Yin, S.; Zhang, H.; Sun, Q.; Zhao, Y. Muscarinic acetylcholine receptor M5 is involved in spermatogenesis through the modification of cell-cell junctions. Reproduction 2021, 162, 47–59.
- Porto, C.; Lucas, T.; Siu, E.; Royer, C.; Trindade, E.; Nader, H.; Lazari, M. Muscarinic Acetylcholine Receptors: Relevance to Infertility and Male Contraception. Immunol. Endocr. Metab. Agents Med. Chem. 2008, 8, 42–50.
- Avellar, M.C.; Siu, E.R.; Yasuhara, F.; Marostica, E.; Porto, C.S. Muscarinic acetylcholine receptor subtypes in the male reproductive tract: Expression and function in rat efferent ductules and epididymis. J. Mol. Neurosci. 2010, 40, 127–134.
- Avellar, M.C.; Lazari, M.F.; Porto, C.S. Expression and function of G-protein-coupled receptors in the male reproductive tract. Acad. Bras. Cienc. 2009, 81, 321–344.
- Xie, M.J.; Ishikawa, Y.; Yagi, H.; Iguchi, T.; Oka, Y.; Kuroda, K.; Iwata, K.; Kiyonari, H.; Matsuda, S.; Matsuzaki, H.; et al. PIP3-Phldb2 is crucial for LTP regulating synaptic NMDA and AMPA receptor density and PSD95 turnover. Sci. Rep. 2019, 9, 4305.
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120.
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349.
- Bray, C.; Son, J.H.; Kumar, P.; Meizel, S. Mice deficient in CHRNA7, a subunit of the nicotinic acetylcholine receptor, produce sperm with impaired motility. Biol. Reprod. 2005, 73, 807–814.
- Arican, E.Y.; Gokceoglu Kayali, D.; Ulus Karaca, B.; Boran, T.; Ozturk, N.; Okyar, A.; Ercan, F.; Ozhan, G. Reproductive effects of subchronic exposure to acetamiprid in male rats. Sci. Rep. 2020, 10, 8985.
- Wessler, I.K.; Kirkpatrick, C.J. Non-neuronal acetylcholine involved in reproduction in mammals and honeybees. J. Neurochem. 2017, 142 (Suppl. S2), 144–150.
- Bishop, M.R.; Rama Sastry, B.V.; Stavinoha, W.B. Identification of acetylcholine and propionylcholine in bull spermatozoa by integrate pyrolysis, gas chromatography and mass spectrometry. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1977, 500, 440–444.
- Saiko, A. Physiological importance of acetylcholine in sperm cytoplasm. Fiziol. Zhurnal 1969, 15, 537–542.
- Schirmer, S.U.; Eckhardt, I.; Lau, H.; Klein, J.; DeGraaf, Y.C.; Lips, K.S.; Pineau, C.; Gibbins, I.L.; Kummer, W.; Meinhardt, A.; et al. The cholinergic system in rat testis is of non-neuronal origin. Reproduction 2011, 142, 157–166.
- Ramirez-Reveco, A.; Villarroel-Espindola, F.; Rodriguez-Gil, J.E.; Concha, I.I. Neuronal signaling repertoire in the mammalian sperm functionality. Biol. Reprod. 2017, 96, 505–524.
- Sliwa, L. Chemotaction of mouse spermatozoa induced by certain hormones. Arch. Androl. 1995, 35, 105–110.
- Sastry, B.V.; Janson, V.E.; Chaturvedi, A.K. Inhibition of human sperm motility by inhibitors of choline acetyltransferase. J. Pharmacol. Exp. Ther. 1981, 216, 378–384.
- Sato, T.; Ban, Y.; Uchida, M.; Gondo, E.; Yamamoto, M.; Sekiguchi, Y.; Sakaue, A.; Kemi, M.; Nakatsuka, T. Atropine-induced inhibition of sperm and semen transport impairs fertility in male rats. J. Toxicol. Sci. 2005, 30, 207–212.
- Ban, Y.; Sato, T.; Nakatsuka, T.; Kemi, M.; Samura, K.; Matsumoto, H.; Cukierski, M.; Zwieten, M. Impairment of male fertility induced by muscarinic receptor antagonists in rats. Reprod. Toxicol. 2002, 16, 757–765.
- da Silva Junior, E.D.; de Souza, B.P.; Rodrigues, J.Q.; Caricati-Neto, A.; Jurkiewicz, A.; Jurkiewicz, N.H. Functional characterization of acetylcholine receptors and calcium signaling in rat testicular capsule contraction. Eur. J. Pharmacol. 2013, 714, 405–413.
- Nieto-Ceron, S.; Vargas-Lopez, H.; Perez-Albacete, M.; Tovar-Zapata, I.; Martinez-Hernandez, P.; Rodriguez-Lopez, J.N.; Cabezas-Herrera, J. Analysis of cholinesterases in human prostate and sperm: Implications in cancer and fertility. Chem. Biol. Interact. 2010, 187, 432–435.
- Radhakrishnan, R.K.; Ravichandran, S.; Sukesh, A.; Kadalmani, B.; Kandasamy, M. Single injection of very mild dose botulinum toxin in the vastus lateralis improves testicular spermatogenesis and sperm motility in ageing experimental mice. Lab. Anim. Res. 2022, 38, 7.
- Dressler, D.; Saberi, F.A. Botulinum toxin: Mechanisms of action. Eur. Neurol. 2005, 53, 3–9.
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the Factors Involved in Male Infertility: A Prospective Review. Int. J. Gen. Med. 2020, 13, 29–41.
- Jin, J.M.; Yang, W.X. Molecular regulation of hypothalamus-pituitary-gonads axis in males. Gene 2014, 551, 15–25.
- Kaprara, A.; Huhtaniemi, I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 2018, 86, 3–17.
- Aghazadeh, Y.; Zirkin, B.R.; Papadopoulos, V. Pharmacological regulation of the cholesterol transport machinery in steroidogenic cells of the testis. Vitam. Horm. 2015, 98, 189–227.
- Carrageta, D.F.; Guerra-Carvalho, B.; Spadella, M.A.; Yeste, M.; Oliveira, P.F.; Alves, M.G. Animal models of male reproductive ageing to study testosterone production and spermatogenesis. Rev. Endocr. Metab. Disord. 2022, 1–20.
- Tilbrook, A.; Clarke, I.J. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol. Reprod. 2001, 64, 735–742.
This entry is offline, you can click here to edit this entry!
