Intestinal microorganisms are composed of bacteria, archaea, eukaryotes, and viruses, and more than 99% of them are bacteria. Approximately 1014 bacteria are known to constitute the intestinal flora in the adult gut, and this number is 10 times the number of human somatic cells.
- intestinal flora
- homeostatic imbalances
- diseases
1. Introduction
The intestinal flora co-exists harmoniously with the host, participate in the digestion and the absorption of nutrients, and also help to maintain the integrity of the host's immune system so as to prevent pathogen colonization [1]. Additionally, intestinal flora consists of various bacteria in low or high abundance, which co-evolve with the host. While the host provides nutrients and a suitable survival place for the intestinal flora, the intestinal flora assists the host in absorbing nutrients, such as vitamins and short-chain fatty acids, in a more efficient manner in order to drive growth processes and to support the functions of the intestinal system and the immune system [2]。
2. Architecture and Composition of the Intestinal Flora
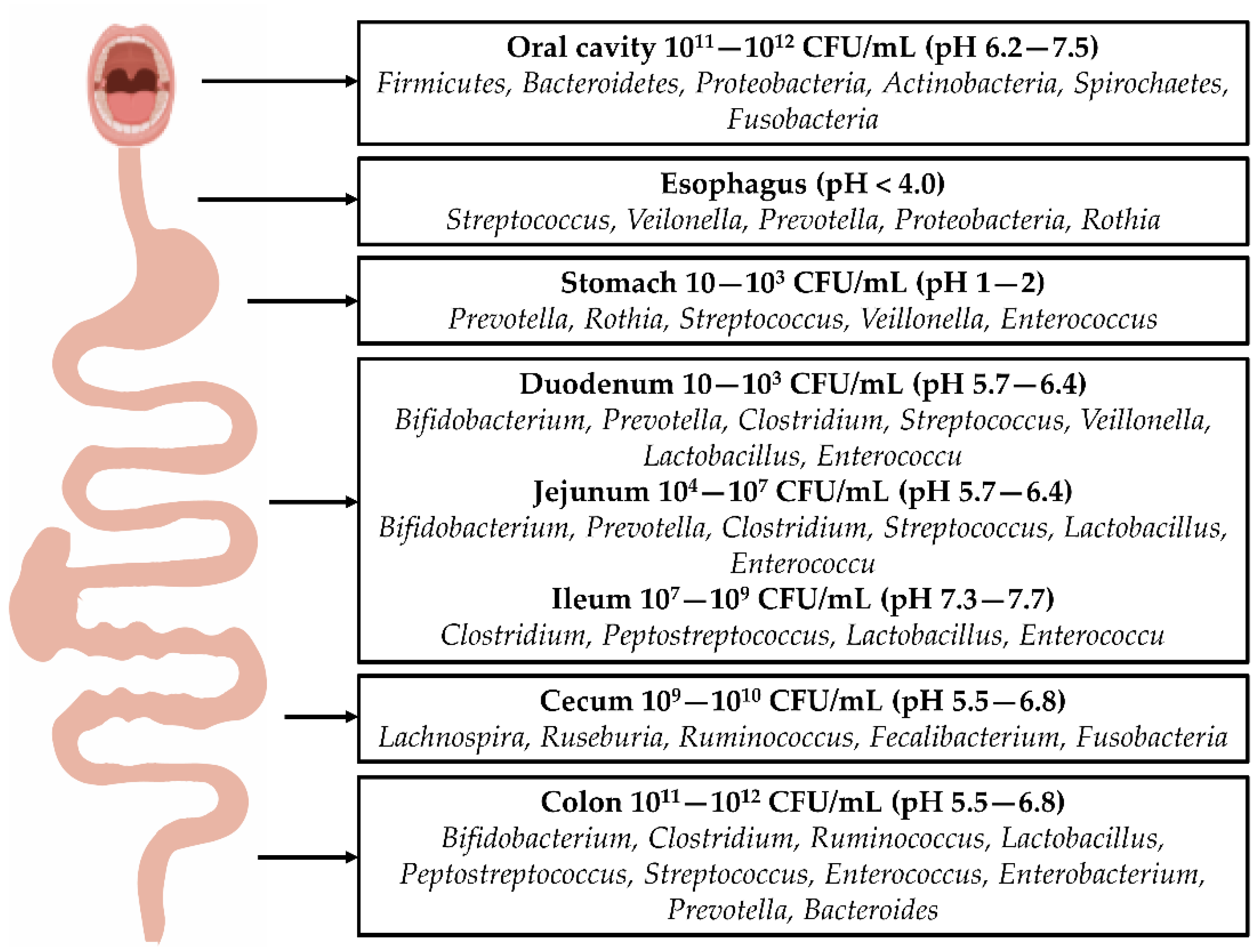
Figure 1. Distribution of gastrointestinal bacteria: The distribution of intestinal bacteria in the digestive tract varies, and there are many types and quantities of bacteria in the oral cavity. Following their entry into the esophagus, the colonization of bacteria is reduced. Due to the secretion of gastric acid, most bacteria in the stomach cannot survive, allowing more acid-tolerant bacteria, such as Prevotella, Roche, and Streptococcus, to dominate. The number of bacteria increases from the duodenum to jejunum and ileum. These bacteria include Clostridium, Lactobacillus, and Enterococcus. A large number of bacteria exist in the colon, including Bifidobacterium, Clostridium, Ruminococcus, Bacteroides, Streptococcus, and Prevotella.
Table 1. Classification of bacterial species in the intestinal flora: According to classification by natural properties, intestinal bacteria can be divided into six categories for the most part: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia. Each category includes bacterial species.
|
Phylum |
Class |
Order |
Family |
Genus |
Species |
|
Firmicutes |
Clostridia |
Clostridiales |
Clostridiaceae |
Faecalibacterium |
Faecalibacterium prausnitzii |
|
Clostridium |
Clostridium spp. |
||||
|
Lachnospiraceae |
Coprocococcus |
Coprococcus eutactus |
|||
|
Peptostreptococcaceae |
Peptostreptococcus |
Peptostreptococcus anaerobius |
|||
|
Veillonellaceae |
Veillonella |
Veillonella parvula |
|||
|
Bacilli |
Lactobacillales |
Lactobacillaceae |
Lactobacillus |
Lactobacillus acidophilus |
|
|
Enterococcaceae |
Enterococcus |
Enterococcus faecalis |
|||
|
Bacillales |
Listeriaceae |
Listeria |
Listeria iuanuii |
||
|
Bacteroidetes |
Flavobacteria |
Flavobacteriales |
Flavobacteriaceae |
Flavobacterium |
|
|
Bacteroidetes |
Bacteroidales |
Bacteroidaceae |
Bacteroides |
Bacteroides fragilis |
|
|
Bacteroides caccae |
|||||
|
Bacteroides pyogenes |
|||||
|
Porphyromonadaceae |
Porphyromonas |
|
|||
|
Parabacteroides |
Parabacteroides distasonis |
||||
|
Rikenellaceae |
Alistipes |
Alistipes finegoldii |
|||
|
Prevotellaceae |
Prevotella |
Prevotella spp. |
|||
|
Proteobacteria |
Gamma proteobacteria |
Enterobacteriales |
Enterobacteriaceae |
Escherichia |
Escherichia coli |
|
Enterobacter |
Enterobacter areogenes |
||||
|
Delta proteobacteria |
Desulfovibrionales Desulfobacterales |
Desulfovibrionaceae Desulfobacteraceae |
Desulfovibrio |
Desulfovibrio intestinalis |
|
|
Desulfobacter |
|
||||
|
Epsilon proteobacteria |
Campylobacterales |
Helicobacteraceae |
Helicobacter |
Helicobacter pylori |
|
|
Actinobacteria |
Actinobacteria |
Actinomycetales |
Actinomycetaceae |
Actinobaculum |
|
|
Corynebacteriaceae |
Corynebacterium |
Corynebacterium glutamicum |
|||
|
Bifidobacteriales |
Bifidobacteriaceae |
Bifidobacterium |
Bifidobacterium adolescentis |
||
|
Bifidobacterium longum |
|||||
|
Fusobacteria |
Fusobacteria |
Fusobacteriales |
Fusobacteriaceae |
Fusobacterium |
Fusobacterium nucleatum |
|
Verrucomicrobia |
Verrucomicrobiae |
Verrucomicrobiales |
Verrucomicrobiaceae |
Akkermansia |
Akkermansia muciniphila |
This entry is adapted from the peer-reviewed paper 10.3390/ijms23158343
References
- Albhaisi, S.A.M.; Bajaj, J.S.; Sanyal, A.J. Role of gut microbiota in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G84–G98.
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14.
- Milani, C.; Ferrario, C.; Turroni, F.; Duranti, S.; Mangifesta, M.; van Sinderen, D.; Ventura, M. The human gut microbiota and its interactive connections to diet. J. Hum. Nutr. Diet. 2016, 29, 539–546.
- Tanaka, M.; Sanefuji, M.; Morokuma, S.; Yoden, M.; Momoda, R.; Sonomoto, K.; Ogawa, M.; Kato, K.; Nakayama, J. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes 2020, 11, 205–216.
- Adlerberth, I.; Wold, A.E. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238.
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40.
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36.
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493.
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040.
- Gonzalez Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143.
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324.
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial biota in the human distal esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255.
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803.
- Nardone, G.; Compare, D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260.
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693.
- Weaver, K.E. Enterococcal Genetics. Microbiol. Spectr. 2019, 7, 7.2.11.
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138.
- Krawczyk, B.; Wityk, P.; Galecka, M.; Michalik, M. The Many Faces of Enterococcus spp.-Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900.
- Murphy, E.C.; Frick, I.M. Gram-positive anaerobic cocci—Commensals and opportunistic pathogens. FEMS Microbiol. Rev. 2013, 37, 520–553.
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 2018, 8, 4.
