Mycetoma has manifested for many centuries. The clinical features of mycetoma were vividly described as “
Padavalmika” or “anthill foot” [
1], an “ant hill-like foot,” in ancient Indian religious book
Atharva Veda (knowledge storehouse of
atharvāṇas, the procedures for everyday life) [
2]. It was Gill, in 1842, who first described multiple cases, in detail, in an ancient southern Indian city, Madura (changed to Madurai in 1949), and named it “Madura foot” [
3,
4]. Carter established fungi as the cause of the disease and used “mycetoma” to describe the tumorous enlargement in 1860 [
5]. Based on the causative pathogens, in 1913, Pinoy first divided mycetoma into two basic types: “Actinomycosis” (caused by the
Actinomyces group) and “True eumycetoma” (caused by true fungi or molds) [
6].
It is clear now that mycetoma is a chronic subcutaneous, granulomatous infection caused by either fungi (eumycetoma) or bacteria (actinomycetoma) living in the soil and water. The bacterial and fungal pathogens get into subcutaneous tissue through broken skin barrier, locally incubate and proliferate, and then progress to visible clinical lesions. Typically, mycetoma presents as a triad of painless subcutaneous tumor-like swelling, multiple sinuses and fistulas, and discharged grains in pus [
7]. If untreated, the pathogens can spread through muscular fascial spaces, or lymphatics, into muscles and bones, and lead to severe consequences including disfiguration, deformity, disability, amputation, and even death [
8,
9], as well as causing heavy economic and medical burdens to social communities [
3,
9,
10].
2. Epidemiology
Mycetoma mainly affects poorer populations in remote rural areas in tropical and subtropical countries at altitudes between 30° North and 15° South, the so-called “mycetoma belt” regions, including Sudan, Somalia, Senegal, Yemen, India, Mexico, and Venezuela [
11,
13,
14]. Other countries with reported mycetoma include Egypt, Mauritania, Kenia, Niger, Nigeria, Ethiopia, Chad, Cameroon, Djibouti, and Somalia in Africa; Colombia, Argentina, and Brazil in Latin America [
11]. Most of the patients have not received enough attention, due to their living in remote rural areas that lack enough medical services, including well-trained medical personnel, diagnostic facilities, and treatment options [
15]. Mycetoma is, therefore, recognized as a “neglected tropical disease” by the World Health Organization (WHO) [
16]. A small number of cases have been reported in other countries, including United States of America, Israel, Germany, The Netherlands, Turkey, Lebanon, Saudi Arabia, Iran, Philippines, Japan, Sri Lanka, and Thailand [
3,
11,
17,
18].
The global incidences of eumycetoma and actinomycetoma are not equally distributed. Globally, eumycetoma accounts for 40% of mycetoma cases, while actinomycetoma accounts for 60% of them [
14]. Eumycetoma is endemic in drier regions, such as Africa, especially in Sudan. These regions are known for their harsh climate with short rainy and long dry seasons and with daily temperatures fluctuating from 45–60 °C to 15–18 °C, which favors the survival of the causative pathogens where fungal infections (
Madurella mycetomatis) are accountable for more than 70% of mycetoma patients [
11]. Actinomycetoma is more prevalent in humid and hot regions, such as South and Central America [
3]. In Mexico, 92% of mycetoma cases are actinomycetoma, mainly caused by
Nocardia (78%) and
Actinomadura madurae (9%), and only 8% of the cases are eumycetoma [
13].
Mycetoma predominantly occurs in men, with the ratio of men to women being 3–5:1. Any age group can be affected, but most of the patients are young adults, between 15 and 40 years of age [
3,
9], the major labor forces conducting farming, herding, and other outdoor activities; especially those working bare-footed are commonly infected. Patients with poor hygiene, malnutrition, and diabetic disease and those suffering with severe dysfunction of the human immune system are also predisposed to these types of infections [
21,
22,
23,
24].
3. Pathogenesis
The pathogens, including both bacteria and fungi, found in soil and water enter the human body through broken skin, caused by either thorn pricks, wood splinters, or accidental cuts, and inoculate and proliferate in the subcutaneous tissues, eliciting local and systemic inflammatory reactions from the host [
3,
31]. In the early stage, a small, painless nodule is formed at the site of injury. With the constant proliferation of bacteria or fungi, the body responds with the accumulation of neutrophils and the release of cytokines and a variety of enzymes; the subcutaneous tissues are then digested, creating a viscous, purulent, or serosanguinous fluid, leading to subcutaneous swelling and increased pressure, eventually breaking down the skin barrier, forming the draining sinuses, and discharging grains with different colors, sizes, and textures (depending on the species of the infecting bacteria or fungi). With the extrusion of the pathogens, the subcutaneous tissues begin a healing process, and the unextruded pathogens (either fungi or bacteria) initiate a new infection, invading uninfected surrounding tissues. The cycle of infection, healing, and reinfection continues without treatment. In the late stage, the infection leads to the causative microorganisms spreading through the fascial planes to the underlying muscles and bones, and consequently, to the destruction and deformity of the osseous and muscular tissues and disfiguration. Mycetoma is accordingly classified as “implantation mycoses” [
32].
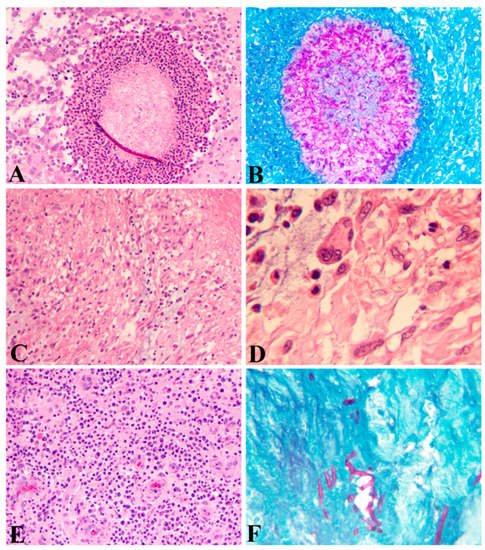
Histopathologically, the local-host reactions to the infections result in the formation of epithelioid granulomas with either fungi or bacteria inside and with surrounding acute and chronic inflammation. Three types of host-tissue reactions against the mycetoma pathogens have been described that form the basis for histological diagnosis and differential diagnosis. Type I: The fungal elements are surrounded by a layer of neutrophils and then surrounded by plasma cells, macrophages, lymphocytes, neutrophils, eosinophils, and blood vessels, forming a granulomatous tissue, which is further surrounded by a fibrous tissue as the utmost outside layer (
Figure 1A), a featured phenomenon called Splendore–Hoeppli. The fungal elements can be stained with PAS (
Figure 1B). Type II: Most of the neutrophils are replaced by macrophages, plasma cells, lymphocytes, and multinucleated giant cells (
Figure 1C,D). Type III: Formation of epithelioid granulomas, containing Langerhans giant cells and scattered fungal elements among granulomatous tissues (
Figure 1E,F) [
33,
34]. These three types of histological manifestations are not separated events but reflect the different stages of the pathogenesis from acute to chronic infection.
Figure 1. Histopathology of eumycetoma. (A) Fungal elements surrounded by a layer of neutrophils and then surrounded by plasma cells, macrophages, lymphocytes, eosinophils, and blood vessels (HE; A; 100 × 1). (B) Periodic Acid Schiff (PAS) stain showing fungal elements (100 × 1). (C) (HE; 100 × 1) and (D) (HE; 400 × 1) Macrophages and Langerhans giant cells predominant in a granuloma. (E) Epithelioid granulomas containing lymphocytes, plasma cells, and epithelioid cells, and proliferated blood vessels (HE; 100 × 1). (F) PAS stain showing a small number of fungal elements in a granuloma (PAS; 100 × 1).
Host systemic reactions to fungi or bacteria include innate and adaptive responses. As with other microorganisms, innate responses, including direct antifungal or antibacterial effector activities and macrophage-mediated phagocytosis, are non-specific, whereas adaptive immune responses, including the activation of specific T cells and the production of pro-inflammatory mediators such as chemokines and cytokines, are specific. Serum Th-1 cytokines include IFN-γ, TNF-α, and IL-2, and Th-2 cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13 in patients infected with
Madurella mycetomatis were found to be increased as compared with the control group [
23]. Th1 cells and associated cytokines are mainly involved in the cell-mediated immunity and activation of the phagocytes for phagocytosis, whereas the Th2 cells and associated cytokines play important roles in the activation and differentiation of B cells to plasma cells for antibody production as well as the generation of memory B cells [
35,
36,
37]. These reactions correspond to the increased aggregation of neutrophils, lymphocytes, macrophages, and plasma cells surrounding either fungi or bacteria as observed in HE slides (
Figure 1A,B).
Increased serum levels of IL8, NO synthase 2 (NOS2), CC Chemokine ligand 5′ (CCL5), and IL10 in mycetoma patients have been linked to genetic alterations in
IL8 (
CXCL8),
NOS2,
CCL5, and
IL10, as demonstrated by analyses of either single-nucleotide polymorphisms or polymerase-chain-reaction and restriction-fragment-length polymorphisms (PCR-RFLPs) in mycetoma patients [
38,
39].
Nutritional factors play multiple essential roles in normal cell growth, metabolism, immune function, and inflammatory responses to pathogen invasion. Macronutrients including carbohydrates, proteins, fats, and liquids, as well as micronutrients including amino acids, vitamins, and minerals, are required for the formation and proper function of leukocytes, lymphocytes, and macrophages. Protein and fat deficiencies lead to impaired collagen synthesis, angiogenesis, fibroblast proliferation, reduced production of cytokines, and chemokines including prostaglandins, thromboxane, and leukotrienes in the inflammatory response to invading pathogens. Deficiencies of vitamin A and Zinc result in altered B-cell and T-cell functions and antibody production, decreased cytotoxicity of natural killer cells, impaired phagocytosis of macrophages and neutrophils, and decreased numbers of granulocytes [
40,
41]. In mycetoma endemic regions, malnutrition in these patients leads to decreased immunity, increased susceptibility to infectious pathogens, including bacteria and fungi, and difficulty in mounting an effective immune response to resolve the infection.
4. Treatment
Treatments for eumycetoma and actinomycetoma are different [
8]. Eumycetoma is treated with antifungal agents in combination with surgical excision, whereas actinomycetoma is treated with antibacterial agents. No specific antifungal medications have been developed for eumycetoma. Currently, recommended agents for eumycetoma include itraconazole (400 mg daily or 200 mg twice daily) [
8], voriconazole (400 mg daily) [
65,
66], posaconazole (800 mg daily) [
67], and terbinafine [
68], individually or in combination. Ravuconazole was recently shown to be effective against
M. mycetomatis in an in vitro study [
33]. Fosravuconazole was developed to treat onychomycosis [
69,
70]. It can rapidly convert to ravuconazole in the body once taken orally. A clinical trial to treat eumycetoma with fosravuconazole at Mycetoma Research Center in Khartoum, Sudan, has been conducted since 2017, with promising results. This could add a new potent drug to treat eumycetoma.
Surgical options for eumycetoma include wide local excision, amputation, and debridement [
12,
71]. Surgical intervention is most suitable for localized lesions, less than 5 cm, or for patients not responding to the aforementioned antifungal therapy. For moderate (>5–10 cm), giant (>10 cm), or multiple lesions involving the bones, itraconazole is initially given for six months to arrest the fungal growth, which reduces the size of the mass(es) and promotes the proliferation of fibroblasts to encapsulate granulomatous tissues. This allows for the surgical excision of the then enclosed lesion, which avoids extensive or multiple surgical procedures [
72]. Itraconazole should be continuously given another six months or longer depending on the treatment efficacy. The clinical outcome is not optimistic. The recurrent rate reached 27.2% in an analysis of 1013 patients receiving a combined therapy of itraconazole and surgery [
73].
Actinomycetoma is usually treated with antibacterial agents. Several medications including amikacin, dapsone (diaminodiphenyl sulphone), trimethoprim-sulphamethoxazole (T-S), and streptomycin sulphate are used in different combinations to treat actinomycetoma, depending on the infecting species.
Actinomadura madurae are treated with streptomycin sulphate and dapsone;
Nocardia spp. with T-S and dapsone; and in
Streptomyces somaliensis and
Actimomyces pelletieri with T-S. Amikacin should be added to T-S and dapsone to treat severe actinomycetoma [
4,
47]. The current recommended regimen for actinomycetoma is a combined therapy of amikacin and T-S in a five-week cycle. The patients are treated with amikacin sulphate 15 mg/kg/day intramuscularly (divided into two daily doses) for 3 weeks and simultaneously with T-S 8/40 mg/kg/day orally for 5 weeks, up to four cycles, depending on the patient’s response. This treatment leads to 90% cure results [
74]. Audiometry and creatinine clearance should be conducted before and after each cycle of amikacin sulphate treatment to monitor ototoxicity and nephrotoxicity.
Other antibiotics, including carbapenems such as imipenem and meropenem, amoxicillin-clavulanic acid, clindamycin, and quinolone, are used in resistant cases [
47]. Surgical excision in combination with medical treatment is needed to treat recalcitrant actinomycetoma [
75]. The durations of treatment for both eumycetoma and actinomycetoma are usually one–two years for resolution.