Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Alzheimer’s disease (AD) is an irreversibly progressive neurodegenerative disease afflicting the elderly, accompanied by devastating cognitive and memory impairment caused by characteristic neuronal and synaptic loss and cortical and hippocampal atrophy. It is hallmarked by the accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles. The underlying mechanisms contributing to the development of the disease remain elusive and controversial.
- Alzheimer’s disease
- nasal discharge fluid
- body fluid
- biomarker
- diagnosis
- non-invasive
- peripheral
1. Conventional AD Body Fluid Biomarkers
A biomarker indicates the change of cells and tissues, which illustrates altered body conditions [16]. In the clinics, biomarkers represent the pathological status and monitor the disease progression. Appropriate methods to detect disease biomarkers in living patients are important for establishing early intervention times and evaluating clinical therapeutic efficacy.
Given that AD is progressive and incurable, an ideal method to detect AD biomarkers is required for AD-specific early detection, economic accessibility, and non-invasive sample collection [17]. The neuropsychological test is a worldwide and classic method to identify cognitive impairment, but multiple profiling is demanded to confirm AD from other neurodegenerative diseases. Although brain imaging (e.g., fMRI, FDG-, amyloid-, and tau-PET) observes disease-specific pathophysiology, the patients may find it difficult to perform neuroimaging tests due to their repeatedly high costs. Recently, observing amyloid-β and tau as biomarkers in CSF and plasma are approved by NIA-AA [14]. In addition to CSF and blood, body fluids that can closely reflect the patient’s pathological condition have been studied. Figure 1 illustrates body fluids that have the potential to be matrices of biomarker detection.
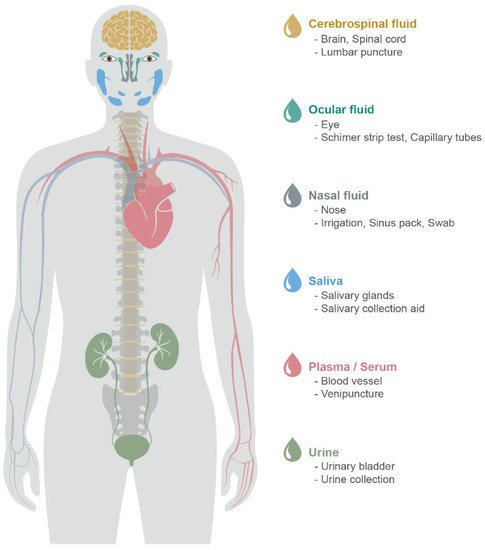
Figure 1. Body fluids for the identification of potential biomarkers for AD. Sample type, organ, sampling method (from above).
1.1. CSF
CSF is a clear and colorless body fluid in the subarachnoid space and circulates within the ventricular system of the brain and spinal cord to supply nutrients and chemicals, remove waste products, and provide the brain immunological protection and mechanical support [18,19]. CSF is produced in the choroid plexus of the brain’s ventricles and reabsorbed into venous sinus blood via the arachnoid granulations. The total volume of CSF is approximately 125–160 mL. CSF is replaced four to five times and regenerates about 500 mL every day [20]. Since CSF reflects biochemical and environmental changes within the central nervous system, CSF is an ideal and useful candidate for detecting potential neuropathology biomarkers [21,22]. CSF is usually obtained by a procedure called a lumbar puncture. The standardized collection protocol of lumbar puncture is carried out under sterile conditions by inserting a needle into the subarachnoid space between the third and fifth lumbar vertebrae [23].
NIA-AA and International Working Group (IWG) 2 have recognized the significance of CSF biomarkers, including Aβ42, T-tau, and P-tau, and incorporated them into diagnostic criteria for AD and MCI [24,25,26]. In AD, Aβ42 concentration and Aβ42/Aβ40 ratio are reduced, and T-tau and P-tau concentrations increase in CSF [18,27,28,29].
Although lumbar puncture is the most common and recommended method for CSF collection, there are some issues: lumbar puncture results in discomfort and pain due to the larger and longer needle and the possibility of CSF contamination by anesthesia. In addition, it is very difficult and expensive to perform the procedure on the subjects repeatedly [23].
1.2. Blood (Plasma and Serum)
Blood plasma is the liquid component in which blood cells are suspended [30]. It delivers nutrients and oxygen to the cells and transports cellular metabolic products. It amounts to about 55% of the total blood volume and is mostly water. Blood plasma is circulated through the body via blood vessels by the pumping of the heart. Functions of blood plasma are maintenance of the blood pressure, pH, immunity, and transportation of electrolytes, nutrients, clotting factors, carbon dioxide, oxygen, other waste products, and excretory proteins.
Blood serum is blood plasma without clotting factors such as fibrinogens [30]. Blood serum includes all electrolytes, antibodies, antigens, hormones, and other substances, except white blood cells, red blood cells, platelets, and clotting factors [31,32]. Blood serum is obtained by coagulation, which allows for clotting of the blood. Both plasma and serum are commonly used for proteomic analysis.
Blood samples are collected by venipuncture, and the protocol commonly used is based on the Human Plasma Proteome Project (HPPP) by the Human Proteome Organization (HUPO) and the National Institute of Health [33,34].
The conventional biomarkers of AD, such as Aβ42, Aβ40, T-tau, and P-tau, are commonly utilized as potential candidate screening molecules in blood samples because they can pass the blood–brain barrier (BBB) [35,36,37]. However, the BBB breaks down in AD patients, which leads to an accumulation of blood-derived neurotoxic proteins in the brain [38].
Biomarkers for neuropathology from blood samples have been controversial because blood communicates with the brain through the BBB, lymphatic vessel, and lymphatic system, which indirectly interchange the materials and substances from the brain into blood, resulting in lower sensitivity and specificity than CSF [35,39,40,41].
2. Novel Peripheral Body Fluid Biomarkers
Recent research has shown that other peripheral body fluids, such as nasal discharge, tear, saliva, and urine, may represent a potential source of biomarkers for neurodegenerative diseases. These peripheral body fluids have advantages over CSF or blood since the collection methods are less invasive and enable low-cost biomonitoring.
2.1. Nasal Discharge
The occurrence of olfactory deficits, named anosmia or hyposmia, in AD has been characterized for decades, and often these deficits precede the cognitive decline [82,83,84,85]. Olfactory neuropathology is the cause of olfactory dysfunction, and structural and functional evidence supports this view, including abnormal APP processing and neuroinflammation [86,87,88,89,90]. The central olfactory processing regions, such as entorhinal and transentorhinal areas, olfactory bulb, and other medial temporal lobes, anatomically overlap with the regions involved in early AD pathology [82,91,92]. AD postmortem and antemortem studies revealed that the olfactory system shows classic AD hallmarks such as intracellular neurofibrillary tau tangles and amyloid plaques [93,94,95,96,97]. In particular, nasal discharge surrounds the olfactory system and captures the neuropathology occurring in the system, emerging as a potential matrix of fluid biomarkers.
Nasal discharge is a slippery and gelatinous fluid produced by mucous membranes in the olfactory mucosa. Nasal discharge is 95% water, glycoproteins, proteoglycans, lipids, proteins, and DNA. The purpose of nasal discharge is to protect the olfactory epithelium (OE) and the respiratory system by blocking the infections of pathogenic antigens. Nasal discharge fluids serve to humidify and clean inhale air and provide proteins of the innate immune system. Additionally, nasal discharge fluids trap and dissolve odorants for the olfactory receptor neurons.
Since the olfactory system is exposed to the external environment, the collection of nasal discharge fluid is easily accessible and non-invasive.
Early studies identified the presence of amyloid-β peptide and amyloid precursor proteins (APP) in postmortem AD patients’ olfactory mucosa samples [94,102]. Aggregation of amyloid-β expression was detected in 71% of AD cases, 22% in normal cases, and 14% in other neurodegenerative disease cases [103]. Biopsy examination identified Aβ expression from the normal, MCI, and AD subjects [104]. Sampling human olfactory environment for AD-related research advanced from taking autopsy or biopsy samples to collecting human nasal discharge. A study collected nasal smears by swabbing from multiple nasal areas, such as the common nasal meatus, inferior concha, middle nasal meatus, and olfactory cleft [71]. Subsequent studies analyzed Aβ expression in nasal discharge fluid by immunoassay and proved that the level of oligomeric Aβ in nasal discharge was higher in AD than normal [67,105]. A 3-year longitudinal study by Yoo et al. confirmed that the presence of oligomeric Aβ could predict the cognitive decline progression [67].
Similarly, early studies used the histopathological method to detect T-tau in postmortem and antemortem samples of AD patients’ olfactory systems [95,106]. Later, immuno-histochemical studies demonstrated that tau pathology in the olfactory system correlated with AD pathology progression [93,107]. ELISA-analysis of nasal smear swabs indicated that P-tau/T-tau ratios were more significant in AD than control [71,108].
Non-core AD hallmark biomarkers have also been identified in the olfactory system. Expression of α-synuclein was identified in postmortem OE of AD sample [103]. A study showed increased microRNA-206 in AD patients’ OE through qRT-PCR [109]. Proteome analysis was done on nasal discharge samples from young, healthy groups and elderly groups, and identified a list of associated proteins with age variability [110]. However, little is known about the molecular machinery responsible for mucus proteome and its changes in neurodegenerative diseases.
2.2. Tears
Tears have a high protein content and have been widely investigated for biomarker studies for ocular diseases and diabetes [113,114]. Major tear proteins, lipocalin-1 and lactotransferrin, are involved in the inflammatory and immune processes [113,115]. Researchers studying neurodegenerative diseases have also hypothesized that neuroinflammation could be reflected in tear proteins due to the extension of the central nervous system. Recent AD studies conducted tear analyses and discovered the potential of tear biomarkers in AD.
Gijs et al. measured Aβ42 in tears using multiplex immunoassays and found the Aβ42 levels changed with increasing AD stage with an area under the curve (AUC) of 0.725 [118]. Other Aβ peptides, Aβ38 and Aβ40, were also detected in their subsequent study [119]. Recently, Wang et al. developed a biosensor and detected variable Aβ42 levels in different age groups of healthy participants [120]. Gijs et al. also analyzed T-tau, and its levels were also able to discriminate between AD patients and healthy controls with an AUC of 0.81 [118,119]. Quantitative proteomic results profiled that lipocalin-1, dermcidin, lysozyme-C, and lacritin can serve AD biomarkers [121]. One LC/MS evaluation identified elongation initiation factor 4E (elF4E) uniquely in AD tear samples, and a PCR-based analysis showed elevated total microRNA abundance in AD patients’ tears and especially higher microRNA-200b-5p levels in tears of AD patients compared to healthy controls [122].
2.3. Saliva
Saliva is an easily accessible, non-invasive body fluid containing 98% water containing electrolytes, proteins, peptides, hormones, sugar, epithelial cells, white blood cells, enzymes, and lysozymes [123]. The functions of saliva are the protection and maintenance of oral mucosa, digestion, the perception of taste, and the control of microorganisms [124,125]. Saliva is secreted from three major salivary glands, named the sublingual, submandibular, and parotid, and they are innervated by the cranial and facial nerves [126]. The direct innervation of the glossopharyngeal nerve through the otic ganglion suggests that saliva can be a promising candidate of biomarker source for assessing pathological physiologies of the nervous system [127].
Several various methods for collecting saliva have been described in the past years. In 2007, the World Health Organization and International Agency for Research on Cancer described the protocol for saliva proteomics [77].
In the last few years, various studies have detected increased Aβ42 in AD patients’ saliva using sandwich and nanobead ELISAs. Bermejo-Pareja et al. analyzed saliva samples by immunoassays and identified a statistically significant increase in saliva Aβ42 levels in mild AD patients than normal control [128]. Subsequent studies similarly showed elevated Aβ42 levels in AD saliva samples [129,130,131]. In contrast to these findings, other results showed no detection of Aβ42 or Aβ40 in saliva with immunoassays [127,128,132]. On the other hand, a recent study demonstrated decreased Aβ42 level in AD patients’ saliva [133]. Some preliminary tau investigation was carried out in the 2010s, and Shi et al. reported an increased P-tau/T-tau ratio in patients with AD compared to healthy controls [127]. A subsequent study also confirmed this increased P-tau/T-tau ratio in AD versus healthy controls [134]. In contradiction with these findings, results demonstrated no significant difference in salivary T-tau between AD and mild cognitive impairment or healthy controls [135].
A growing number of studies have examined possible biomarker candidates other than Aβ and tau peptides. Lactoferrin, for instance, is a pleiotropic protein with several immunological properties, including antibacterial, antiviral, antioxidant, and anti-inflammatory functions [136,137]. The first investigations on salivary lactoferrin found a decreased lactoferrin level in AD patients’ saliva compared to healthy controls [138]. A more recent study supported this finding by comparing salivary lactoferrin levels with amyloid-PET neuroimaging data [139]. Acetylcholinesterase degrades acetylcholine neurotransmitters released into the synaptic cleft and terminates acetylcholine neurotransmission, and PET study results demonstrated decreased acetylcholinesterase catalytic activity in AD patients’ brain regions [140,141,142]. An initial report on the salivary acetylcholinesterase activity was carried out by Sayer et al. and showed a significant decrease in AD patients [143]. Further studies also suggested a reduced salivary acetylcholinesterase activity in AD patients [144,145]. Protein carbonyl levels result from protein oxidation, and multiple studies examined elevated protein carbonyls in brain regions of AD patients [146,147]. One study evaluated and identified protein carbonyl levels in saliva of AD patients and healthy controls [148]. Metabolomics is an emerging research technique used in various research fields to identify metabolites within a target sample. Yilmaz et al. analyzed saliva samples from healthy control, mild cognitive impairment sufferers, and AD patients. They profiled multiple metabolites that changed significantly in the saliva of MCI and AD patients compared to healthy controls [149].
2.4. Urine
Urine contains thousands of proteins, mostly metabolic wastes, and is currently actively utilized to study pregnancy, aging, and kidney diseases [150,151]. Nevertheless, for many years urine has been neglected as a promising source of biomarkers for studying AD since there is little agreement on urine reflecting the changes occurring in the brain due to the BBB and glomerular filtration. However, several studies have indicated the potential of urinary biomarkers in neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease [152,153,154].
More importantly, urine collection does not require special equipment and can be repeated without discomfort to subjects. The Human Kidney and Urine Proteome Project (HKUPP) in 2005 and European Kidney and Urine Proteomics (EuroKUP) in 2008 were initiated to promote proteomics research, and they together have achieved the establishment of a standard protocol for urine collection and storage [78,79,80,81].
Initial report on detecting Aβ peptide in the urine of AD patients was carried out by Takata et al. by Western blot analysis and suggested that monomeric Aβ level may reflect the severity of AD [155]. A key question raised by Takata et al. was that they could not pinpoint the origin of Aβ in urine. A recent study developed an indirect competitive ELISA to measure Aβ42 in human urine samples [156].
Several studies have profiled potential urine protein biomarkers for AD. One study identified 15 proteins using LC/MS-MS and validated three proteins, SPP1, GSN, and IFGBP7, by ELISA [157]. Higher urinary AD7c-NTP, Alzheimer-associated neural thread protein, was demonstrated in another study [158]. Watanabe et al. analyzed AD patients’ urine samples and profiled 109 proteomes differentially expressed in AD and healthy controls [159]. Their subsequent study showed that apolipoprotein C3 levels in AD patients’ urine samples were higher in the AD and MCI groups than healthy controls using ELISA [160]. Urine also contains many metabolites, reflecting the gut microbiome theory in neurodegeneration studies [161,162,163]. One study recently examined urinary metabolome using NMR spectroscopy and UHPLC-MS and built a model that could discriminate between AD and healthy age-matched controls [164]. Another study developed a new screening approach using LC/MS and proposed that lipid peroxidation compounds may be potential predictors of early AD [165]. In addition, many studies reported microRNA in human urine samples and demonstrated that urinary microRNAs are relatively stable under various storage conditions, supporting their utility as urinary biomarkers [166,167,168].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14081532
This entry is offline, you can click here to edit this entry!
