Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Medicinal
Molecular docking plays a significant role in early-stage drug discovery, from structure-based virtual screening (VS) to hit-to-lead optimization. VS is a computational approach used to identify chemical structures that are predicted to have particular properties. In drug discovery, it involves computationally searching large libraries of chemical structures to identify those structures that are most likely to bind to a target protein.
- molecular docking
- virtual screening
- machine learning
- protein-ligand scoring function
1. Introduction
Structure-based virtual screening (VS), also known as target-based VS, attempts to predict the best interaction of a ligand against a target protein to form a complex and employs scoring functions to estimate the binding affinity of the protein–ligand complex [125]. As a result, all the ligands are ranked according to their binding scores to the target, and the high-scoring ligands are selected for experimental measurement. In recent decades, advances in VS have been made in the following:
- (1)
-
There have been developments in structure-based VS approaches, including improvements in sampling and scoring methods, that have resulted in significant improvements in docking, scoring and screening performances [46].
- (2)
-
Developments in GPU processing speeds and cloud computing have dramatically increased computational power. Researchers are now able to computationally process vast numbers of compounds in the drug-like chemical space.
- (3)
- (4)
-
The number of compounds that are commercially available or can be readily synthesized has grown dramatically in recent years. For example, as of March 2021, the WuXi GalaXi and Enamine REAL Space collections contain 2.1 billion and 17 billion compounds, respectively [129]. In June 2022, the WuXi GalaXI and Enamine REAL Space collections have grown up to 4.4 billion and 22.7 billion compounds, respectively.
The convergence of these breakthroughs has positioned structure-based VS to be a promising direction for the discovery of novel small molecule medicine. With the appropriate computing infrastructure, it becomes practical to virtually screen ultra-large compound library (synthesized or purchasable) to find virtual hit compounds, some of which (usually up to 100 compounds) can be experimentally tested.
2. Molecular Docking Protocol
Molecular docking methods predict receptor–ligand interactions at an atomic level and are widely utilized in structure-based VS. The docking process samples the optimal conformation based on the complementarity between the receptor and the ligand. Figure 1A shows the initially proposed “lock-and-key model”, which refers to the rigid docking of receptor and ligand to find the correct orientation for the “key” to open the “lock”. This model emphasizes the importance of geometric complementarity [18]. However, the real binding process is very flexible whereby the receptor and ligand changes their conformation to complement each other well. As shown in Figure 1B, the induced fit model considers structural flexibility and selects the lowest-energy bound state. Currently, major limitations of docking methods include a restricted sampling of both ligand and receptor conformations in pose prediction, as well as the previously discussed limited accuracy of scoring functions in affinity prediction.
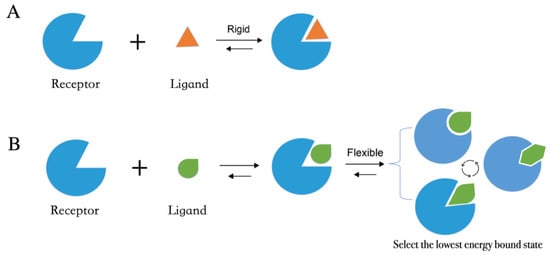
Figure 1. Two models of molecular docking. (A) A lock-and-key model. (B) Induced fit model.
The methods that improve sampling of ligand conformations can be defined as (i) incremental ligand construction, (ii) multiple conformers generation for docking and (iii) stochastic sampling [130]. In the first approach, the ligands are partitioned into small fragments that are individually docked into the receptor pocket according to the geometric fit. Docked fragments are then incrementally assembled to form an entire ligand within the binding pocket [131]. In the second approach, multiple low-energy conformations of the ligand are generated at first, and then individually docked against the receptor pocket [132].
The third and widely used strategy to account for ligand flexibility are stochastic methods, such as Monte Carlo (MC) or genetic algorithm (GA). MC algorithm, also known as simulated annealing, simulates docking by randomly generating minor changes in the position, orientation or conformation to generate new poses that are accepted or rejected based on the Metropolis acceptance algorithm [133]. The modeling begins at a high temperature such that there is a high probability of accepting the next conformation sampled. Then, the temperature is progressively decreased to reduce the conformational freedom of the system and to capture the receptor–ligand complex in a low energy state. GA employs a different approach inspired by Darwin’s theory of evolution [134]. The ligand begins as a random population of position, orientation and conformational states modeled as a set of chromosomes. Then, random crossovers and mutations are performed to produce another set of conformations. The conformation with the lowest binding energies with the receptor is accepted and then used to produce a new generation. This cycle is iteratively repeated until the local energy minimum of the receptor–ligand complex has been reached.
Many proteins possess varying degrees of flexibility, which can range from a slight perturbation of the ligand binding pocket to a complete reconstitution of the pocket. Therefore, an inadequate sampling of protein flexibility can result in an increase of both false positives and false negatives in VS experiments. Several approaches have been developed to tackle the issue of protein flexibility in recent years [135]. One common approach, named “ensemble docking”, is to utilize multiple receptor conformations in docking runs and to select the best-scoring conformation for further investigation [136,137,138]. The receptor conformations are commonly obtained from different X-ray and NMR structures or by sampling structures from molecular dynamics (MD) simulations. For instance, Abagyan and co-workers have investigated strategies for the selection of experimental protein conformations for VS and have found that the use of ensemble conformations of receptors co-crystallized with larger ligands provided the best results [139,140]. However, it has been noted that the use of excessively large numbers of receptor conformers in ensemble docking can lead to an increased number of false positive samples and linearly increased computational costs [135,141]. To alleviate some of these performance issues, ML techniques can be employed to help classify active and inactive compounds following ensemble docking [142]. Chandak and co-workers have tested multiple supervised ML methods trained on the DUD-E database to learn the relationship of a compound’s predicted binding affinities to the classification task.
An alternative approach to account for protein flexibility is to employ “soft docking”, where the interactions between the protein amino acid sidechains and the ligand is iteratively changed to allow partial clashing between the atoms of the protein and ligand [143]. For example, Ravindranath and co-workers have proposed a soft docking program, AutoDockFR [144], which simulates sidechain flexibility by sampling a large number of explicitly specified receptor sidechains and searching for energetically favorable binding poses for a given ligand. AutoDockFR optimizes protein–ligand interactions using the AutoDock4 force field and using a GA method combined with a Solis-Wets local search. This soft docking approach has achieved better binding pose prediction compared to rigid protein docking protocols but has also been associated with an increased number of false positive hits in structure-based VS [145].
3. Workflow in Virtual Screening
Structure-based VS relies on docking of large collections of compounds into the binding pocket of target protein, and then evaluating whether the protein–ligand contacts will drive binding. As shown in Figure 2, the general VS workflow can be as follows:
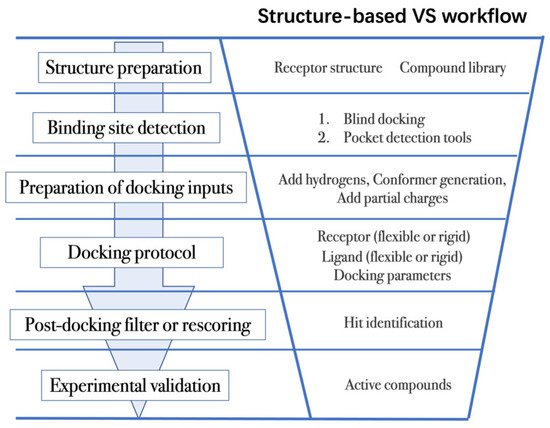
Figure 2. General scheme of a VS workflow.
- (1)
-
The first step is to obtain the 3D structures of a given target as well as the compound library. Experimental determined structures can be readily retrieved from the Protein Data Bank (PDB) [146], in which more than 120,000 unique protein structures have been determined through an enormous experimental effort. However, this represents a small fraction of the billions of known protein sequences whereby the 3D structure of a novel target is usually not available. In order to overcome this limitation, traditional computational prediction methods (such as homolog modelling and ab initio modelling) [147,148], as well as the recently developed DL methods (such as AlphaFold2 and RoseTTAFold) [95,127] can be employed to obtain the 3D structures of target proteins. In addition, the compound library or chemical space used in VS is also vital for hit identification.
As discussed above there is a growing number of options to dock to. It is important to note that the selection of which structure to dock to is not trivial. Docking results will differ depending on the conformation, apo/holo status, and quality of structure. One method, screening performance index, can be used to select good structures to use in prospective VS [149]. This index consists of five calculated terms that describe the docking performance of a set of structures on a set of known active compounds. Their testing has generally indicated that co-crystal structures with large ligands bound score well on the index and can be picked for prospective studies. These methods are limited because they require labeled datasets which may not be available for novel targets.
Compound libraries of approved drugs, natural products, already synthesized or purchasable compounds/fragments are commonly used in VS campaigns [29,130,150]. The well-known ZINC database contains over 750 million purchasable compounds, including over 230 million compounds in ready-to-dock 3D formats [151,152]. Recently, Jiankun and coworkers performed docking-based VS using a ultra-large compound library (more than 100 million compounds from ZINC make-on-demand compounds) to discover inhibitors targeting AmpC β-lactamase and D4 dopamine receptor [29]. Other databases, such as DrugBank [153,154,155] and Human Metabolome Database (HMDB) [156,157,158,159] are used to repurpose the approved drugs or human metabolites to the novel targets.
- (2)
-
The next step is to detect the binding site. Typically, the binding pocket on which to focus the docking calculations is known. For example, the binding site is chosen based on the information of co-crystallized ligand/substrate binding site, such as ATP binding site or protein–protein interactions (PPI) interface. However, when the binding site information is missing or a novel binding pocket needs to be explored, there are two commonly employed approaches, “blind docking” simulation [160,161] and pocket prediction algorithms. The first approach uses docking methods to search over the entire target structure to find a favorable ligand binding site, but it has a high computational cost in sampling. For the second approach, several available software can be employed to detect binding pockets, including AlphaSpace [162,163], FTMap [164], MDpocket [165], Fpocket [166], SiteMap [167] etc. These methods detect concave pockets on the protein surface by characterizing the spatial composition of amino acids or using the chemical probe to find favorable hot spots. Since drug resistance can arise for the orthosteric site of target proteins, these methods can be used to identify additional binding pockets that can be exploited for the design of novel inhibitors, such as allosteric or cryptic pockets [168,169].
- (3)
-
Once the binding site is determined it is important to carefully prepare docking input files to achieve successful VS. The preparation of protein structures starts from the assignment of protonation states for the amino acids, which can be done using software including PROPKA [170], H++ [171], and SPORES [172]. Then hydrogen atoms and partial charges are assigned. A popular software for this task is PDB2PQR [173,174]. In addition, the consideration of water molecules and metal ions can be crucial in certain target structures. Explicit water molecules mediating protein–ligand interactions should be analyzed and can be used to identify water-mediated interactions and avoid incorrect binding poses [175,176,177]. It is also important to consider coordination interactions between metal ions and ligand molecules for metalloprotein complexes [45,178].
Unlike proteins, most compounds used in VS are stored in line notation, such as Simplified Molecular Input Line Entry Specification (SMILES) string [179]. The 3D atomic coordinates of these compounds can be obtained from the line notation using several opensource softwares, such as RDKit and Openbabel [180,181,182], or commercial softwares, such as Omega and ConfGen [183,184,185]. Ligand protonation is also important since it affects the net charge of the molecule and the partial charges of individual atoms. Different docking programs will employ different charge assignment protocols. For example, AutoDock uses Gasteiger-Marsili atomic charges whereas the AutoDock Vina does not require the assignment of atomic charges, since the scoring terms that compose its scoring function are charge-independent [43,186].
- (4)
-
After the input files are created, the appropriate docking protocol must be selected. As has been discussed in Molecular Docking Protocol (Section 4.1), there are many different docking protocols that consider protein and ligand flexibility to enhance the performance of pose prediction. One of the most commonly used protocols is to perform flexible ligand–rigid receptor docking for each docking run, and then dock multiple protein conformations using the ensemble docking strategy [139]. In addition, several docking programs can be combined to avoid the limitations of one algorithm. For instance, Ren and co-workers have explored the effects of using multiple softwares in the pose generation step [187]. They use a RMSD-based criterion to come up with representative poses derived from 3 to 11 different docking programs. The resulting pose prediction achieves better performance than that of each individual docking program.
- (5)
-
Following docking, the results can be rescored or filtered. The computer-generated poses are evaluated based on the ability of the docking protocol to (i) select favorable binding poses for each ligand, and (ii) rank the ligand library to select high scoring hits for experimental measurement. Although the docking calculations are fast enough to process large compound libraries, they suffer from the inherent problem of calculating binding affinities from several simplified scoring terms. One remedy for improving the performance of VS is to employ more rigorous free energy calculations to postprocess docking poses. The main limiting factor in the application of free energy calculations to large chemical libraries is the high computational cost.
In recent years, post-docking filter methods have gained significant interest in drug discovery because they usually provide higher hit rates in VS with low additional computational cost and result in better correlation with experimental data in retrospective benchmarks. Several methods have been designed to eliminate false positive hits obtained from the initial docking experiments. Marcou and co-worker proposed the use of molecular interaction fingerprints (IFP), which are simple bit strings that convert the 3D information of protein–ligand interactions into a 1D vector representation, for the screening of CDK2 inhibitors [188]. The authors demonstrate that using post-docking filters that calculate the Tanimoto similarity of IFP between docked pose and co-crystal pose is more statistically accurate compared to classical scoring functions in discriminating active compounds from inactive ones. They base this on the assumption that active compounds should have certain specific interactions or contacts with their target to display activity. Bertho and co-workers reported a similar post-docking filtering strategy, namely automatically analyzing poses using self-organizing map (AuPoseSOM) to examine the interatomic contacts between the ligand and the target [189]. This type of approach is target-specific and requires the co-crystal ligand pose as the reference. ML can also be applied to this task. Stafford and co-workers introduced AtomNet PoseRanker, a graph CNN trained on PDBbind v2019 to rerank putative co-crystal poses [149].
Another post-docking strategy is the rescoring of docked poses using a consensus model or an advanced ML scoring function. On one hand, the consensus model uses several different scoring functions to re-assess the docking poses generated from a single docking algorithm. Charifson and co-workers have proposed an approach that takes the intersection of the top-scoring molecules according to two or three different scoring functions. They found it provides a dramatic reduction in the number of false positives identified by individual scoring functions on case studies of p38, IMPDH and HIV protease [190]. On the other hand, advanced ML scoring functions developed in recent years, such as AtomNet [109], vScreenML [191], ΔVinaRF20 [101], ΔVinaXGB [102], SIEVE-Score [192] and RF-Score-VS [117], outperform classical scoring functions in screening performance comparisons on benchmark test sets. However, there is no guarantee that ML scoring functions can outperform classical scoring functions on novel targets that are largely different from the samples in the training data set [193].
The above (1) to (5) steps summarize the workflow of VS process. Other structure-based approaches, such as MD simulations, have also been widely utilized in combination with docking to improve VS performance. MD simulations are an efficient approach to discover cryptic binding pockets (in step 2, binding site detection) [169,194], to sample multiple receptor conformations in ensemble docking (in step 4, docking protocols) [136], and to evaluate the interactions of the predicted receptor–ligand complexes (in step 5, post-docking analysis) [195,196].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27144568
This entry is offline, you can click here to edit this entry!
