1. Introduction
The recycling process of plastic can be divided into various types: primary, secondary, tertiary, and quaternary recycling [
15]. Primary recycling is the processing of a specified and uncontaminated material, commonly scrap, from an industrial process. Furthermore, to provide a good product quality, recycled scrap or waste plastics can be mixed with new materials [
16]. Nevertheless, the primary recycling process needs homogeneous, clean, and non-degraded materials, such as packaging, bottles, and pre-consumer products, with the product of primary recycling being quite similar to a virgin one [
17].
The mechanical recycling of plastic waste is secondary recycling; the most common method for recycling plastic waste. Mechanical recycling processes post-consumer plastics, to produce the raw materials for various plastic products [
18]. In comparison, the recycling process depends on the chemical and physical properties of the waste plastic feed, in terms of its origin, composition, and form [
19,
20].
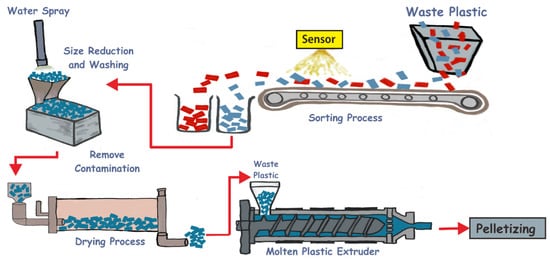
Figure 1 is related to the technology of recycling waste plastics by the mechanical method. Mechanical recycling includes several techniques, for instance, collection, separation, sorting, and washing [
21]. The main objective of the waste plastic sorting process is to obtain high-quality recycled plastic goods, especially from a single polymer stream. Waste sorting technologies are based on various chemical-physical properties of the plastic, for instance, chemical compounds, size, color, and shape. Furthermore, the materials from post-consumer waste contain various polymeric materials and organic substances [
17,
22]. The subsequent process is size reduction. The typical process for size reduction involves cutting or shredding; nevertheless, this process depends on the type of plastic waste stream and plant layout. These processes may occur before or after the sorting stage [
23].
Figure 1. The technology of recycling waste plastic by a mechanical method: sorting process, size reduction, drying process, and molten plastic.
The other processes include size reduction, extrusion, and granulation. These may occur in different sequences and at different times [
19]. The extrusion and granulation processes are required to create a granulation that is possible to convert into flakes. Furthermore, the polymer flakes are typically loaded into an extruder, heated, and pressed through a die, to form a continuous solid polymer product (strand). This can be chilled in a water bath before the pelletized process. The granulation method is often utilized to convert the strands into pellets, which can then be used to produce new products [
23]. To consider the full life cycle of polyurethane foams (PUFs), PUFs were upcycled and reshaped to bulk polyurethanes (PUs) using a transcarbamoylation reaction of up to five cycles. Moreover, four PUFs were prepared and reshaped by compression molding at 160 °C for 30 min, demonstrating the potential of this recycling pathway for PUFs from different origins [
24].
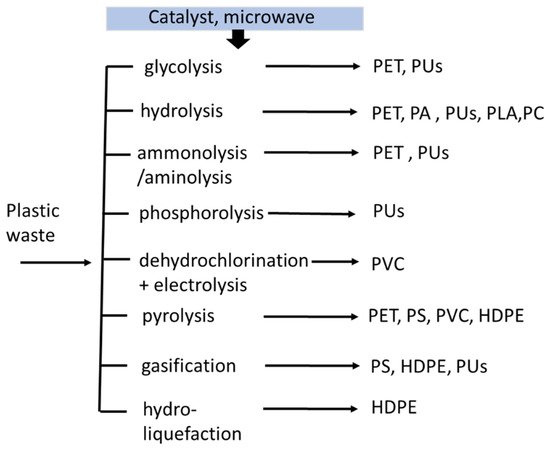
Tertiary or chemical recycling refers to the degradation of polymer bonds. As a result, the recovery of the oligomers monomers produces a smaller molecular weight. Hence, thermoplastic can be obtained with this method [
25]. Some technologies can be applied in the following manner, as shown in
Figure 2:
Figure 2. The chemical recycling route of plastic waste.
- (a)
-
Gasification: the polymer is utilized as a refuse-derived fuel using high temperature. It is converted to syngas with an H2/CO molar ratio of 2:1 in a gasifier; the syngas produced depend on the various polymers.
- (b)
-
Pyrolysis: the plastic waste is converted to pyrolytic oil, which is equivalent to diesel oil. In this chemical recycling, the calorific value of the polymer affects the energy content of the diesel [
26].
- (c)
-
Glycolysis: the ethylene glycol and waste plastic are added in the presence of a catalyst. The long polymer chain is degraded into building blocks, which can be recycled to produce new polymers.
- (d)
-
Hydrolysis: when biopolymers (e.g., PLA) are heated and broken down to their monomer building blocks, they can be dissolved in water. These monomers can be recycled and utilized to make new products [
27,
28].
Quaternary recycling is a method of recovering energy using a combustion process of the waste polymer [
29]. The plastic waste is incinerated. Nevertheless, the released energy is captured and replaced with heat and power. These strategies present a hierarchy of choice, in ascending order, from primary to quaternary, for managing resources and minimizing the processing costs of converters.
2. Recycling Polypropylene
PP has a linear hydrocarbon chain, with a melting temperature of 160 °C [
61]. PP is produced from propylene with a catalyst of metallocene or Ziegler-Natta. Furthermore, PP has excellent thermal, physical, and mechanical properties at ambient temperature. PP is an extensive application in plastics, stationery, furniture, food containers, and automotive industries [
62]. PP recycling can be approached by chemical and mechanical methods, such as pyrolysis and hydrogenolysis. In addition, chemical recycling provides a chance to recycle waste plastic and convert it into higher value-added chemicals, especially for fuel additives. PP recycling approaches can be integrated with chemical refineries and generate a new generation of recyclable-by-design polymers [
63,
64].
Upcycling PP waste by hydrogenation has recently received much attention.
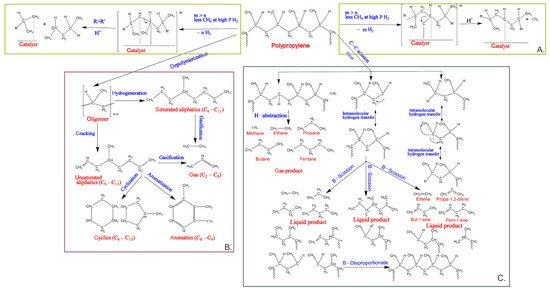
Figure 3A illustrates the pathway of polypropylene degradation by hydrogenolysis. Chen et al. found a depolymerization mechanism of polypropylene under a catalyst by hydrogenolysis. The initial step of the reaction of hydrogenation is dehydration and adsorption. Hence, a hydrogen-depleted intermediate is formed. The hydrogenation of the fragments occurs during C-C cleavage, mainly resulting in new products. The lighter product with a R’-group will desorb more quickly [
65].
Figure 3. The depolymerization mechanism of polypropylene reaction: (
A) hydrogenolysis into gas and liquid alkanes, (
B) supercritical water depolymerization process, and (
C) pyrolysis in a semi-batch reactor under atmospheric pressure [
65,
66,
67].
3. Recycling Polystyrene
PS is an aromatic polymer produced by polymerizing a styrene monomer. PS is a popular material because it has excellent physical properties, such as strength, durability, versatility, and low cost [
102]. PS is extensively used in the form of expanded PS foam, which has a low thermal conductivity, good resistance to many corrosives, and is nearly impervious to moisture [
103]. Styrene polymerization can be achieved through various intermediates and/or active species, such as cationic, coordination polymerization, anionic, and radicals. Free-radical polymerization is mostly used in the commercial production of atactic PS with a higher molecular weight, up to 200,000–300,000 g/mol. It can produce an amorphous polymer with a comparatively high glass transition temperature of Tg = ~100 °C [
104].
PS is difficult to degrade in natural environments. Nevertheless, chemical, mechanical, and thermal recycling are used to degrade the large molecule of PS. One of the methods for chemical recycling is dissolution.
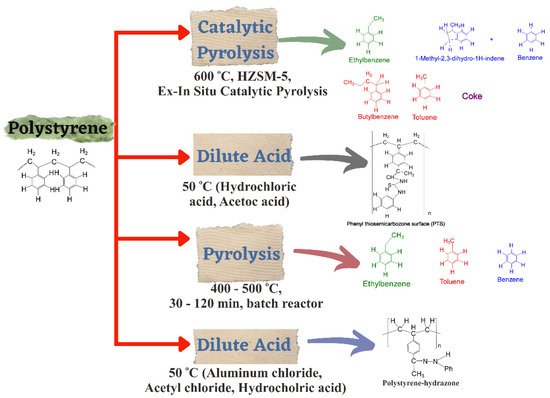
Figure 4 illustrates PS degradation by dissolution and a scheme of liquid products valuable in PS depolymerization, such as catalytic pyrolysis, dilute acid, and pyrolysis [
105,
106]. The polymeric components are first dissolved, then various processes are used to recover the solvent and polymer [
107]. The recycling of foamed polymers by using solvents has several advantages. Filtration can be used to eliminate any insoluble impurities, leaving the polymer clean for any further treatment. Additionally, the dissolution process enables the separation of plastics from other waste and insoluble polymers, according to their chemical structure, a process known as selective dissolution. For expanded materials, dissolving the foam in a suitable solvent results in a significant volume decrease (over 100 times), lowering transportation expenses [
108].
Figure 4. Scheme of valuable liquid products for PS depolymerization for different reaction paths.
Cymene, terpinene, phellandrene, and limonene are used for recycling expanded polystyrene. Furthermore, limonene is an excellent solvent and antioxidant for recycling expanded polystyrene throughout the heating process. A prior work discovered that using d-limonene as a diene compound in the thiolene reaction dissolves PS, allowing it to be recovered from the solution [
101]. Furthermore, p-xylene/n-heptane, methyl ethyl ketone (MEK)/methanol, and MEK/n-hexane are suitable for recycling PS foam. Conventional solvents such as methanol/xylene can dissolve PS at various temperatures. Within a specific temperature range, a rising temperature will contribute to the rapid recovery of PS. Due to environmental damage, a more environmentally-friendly solvent (D-limonene, produced from citrus fruit rinds) was utilized to obtain 100% recycling of expanded polystyrene [
109]. Gil-Jasso studied the application of essential oils to dissolve and recover PS waste. Various solvents were applied, such as chamomile, thyme, star anise, and eucalyptus oil, with a total percentage of PS recovery of more than 95%, and with a reaction time up to 833 s [
110]. Polarity influences the solubility of polymers in solvents. A polymer is naturally inclined to dissolve more readily in the non-polar solvents that are chemically and physically closest to the XPS. Nevertheless, polar solvents can also be utilized in the recycling process if they do not have a strong tendency for hydrogen bond formation [
108].
Pyrolysis and catalytic degradation of PS is a waste treatment process (thermal recycling) that can be utilized as a substitute for landfill disposal [
111]. Simple thermal cracking at low temperatures can convert PS to styrene, without catalysts. PS pyrolysis is primarily influenced by catalyst presence, reaction time, temperature, and reactor type. Products consist primarily of liquid chemicals at low temperatures (mono aromatic). Coke and gas will be increased slightly at higher temperatures, and the liquid fraction includes many aromatics (dimer, trimer) [
112]. Furthermore, adding a catalyst reduces the residence time of polymer degradation in the reactor and decreases the process temperature, by lowering the activation energy by breaking the chain of C–C bonds. PS catalytic depolymerization can be split into acid and alkaline [
113]. Numerous research works on the catalytic pyrolysis of PS have been performed, including metallic oxides (alumina, alumina-silica, CuO/Al
2O
3, BaO, Al
2O
3, SiO
2, K
2O, CaO, or silica), assisted transition metals, mesoporous materials (K
2O−BaO/MCM−4, K
2O/Si−MCM−41, MCM−41 sepiolite derived from nature), as well as clay (pyrophyllite, albite, halloysite, montmorillonite) [
112,
114].
The degradation of PS waste with various basic and acidic catalysts was studied by Anwar et al. The catalytic pyrolysis was conducted with calcium oxide at temperatures ranging from 300 to 350 °C and at atmospheric pressure. The total distillate recovery was up to 77%. On the other hand, a metal carbonate catalyst generated pure styrene. Nevertheless, the yield of styrene was low [
115]. The mechanism of PS catalytic pyrolysis with montmorillonite and albite as a catalyst was investigated before. The first stage of the reaction process was β-scission, followed by intermolecular H transfer, with major products being ethylbenzene and styrene [
114].
4. Recycling of Polyvinyl Chloride
PVC is widely used in extensive applications, such as packaging, construction, electronic industries, and automotive products. Furthermore, PVC has excellent electrical, thermal, mechanical, and chemical resistance properties [
122]. PVC can be degraded with a lower temperature reaction than other plastics. The detailed mechanism of PVC breakdown has been investigated with various models, which were modeled as three processes: (1) converse PVC through several intermediates compounds and HCl; (2) intermediate compounds are degraded into volatile compounds and polyene chains; and (3) polyene breakdown into toluene (and also other aromatics) [
105].
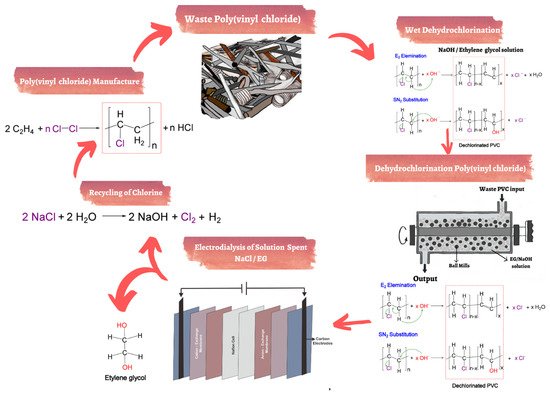
Figure 5 shows how dehydrochlorination and electrodialysis are applied to recycle PVC waste. The recycling of PVC is divided into several parts. The dechlorinating process starts with a mixture of PVC waste and NaOH/ethylene glycol. The PVC waste is dechlorinated and transferred to the EG solution in the form of Cl. Then, the next process is NaCl recovery by electrodialysis with EG solution containing Cl
− and Na
+ through cation and anion exchange membranes [
123]. Furthermore, Kameda et al. studied the electrodialysis of a NaCl/EG solution mixture through ion-exchange membranes. After 5 h, a high desalting ratio was obtained up to 98%. Nevertheless, the Donan effect was decreased by 0.5 wt.% of the efficiency NaCl, with total voltages greater than 4 V [
123].
The other process for recycling PVC is pyrolysis. Nevertheless, PVC pyrolysis has some issues, because this process can produce fuel oil containing a large amount of chlorine [
124]. The Cl
− in fuel oil products of pyrolysis can cause serious corrosion to parts of the machine and transfer toxic chemicals into the environment. As a result, the dichlorination process should be conducted before converting PVC into a high-quality fuel via pyrolysis [
125]. On the other hand, catalytic and noncatalytic pyrolytic processes are used for PVC waste. Catalytic pyrolysis adds a catalyst, to increase the dichlorination process, while adding a sorbent reduces the product’s chlorine compound.
Zakharyan et al. reported treatments of virgin and mixture PVC (multicomponent and binary PVC mixture, chlorine- and bromine-compounds mixtures, biomass, and municipal plastics waste) [
126]. Pan et al. studied chlorine transformation and migration during PVC pyrolysis using TG-FTIR-MS methods. This showed that pyrolysis occurs in two primary steps: the first step of the reaction is using a temperature between 200–360 °C. This phase includes the dichlorination of PVC, which produces a massive amount of benzene and hydrogen chloride. The second step is a reaction temperature of 360–550 °C. The polyethylene chain is broken in the second step, due to a large amount of aromatic organic substances and chlorine-containing compounds [
127]. In addition, the flash pyrolysis of PVC was conducted with a temperature reaction up to 500 °C. The major products were HCl, alkenes, monocyclic aromatics, and PAHs at 3.02%, 2.86%, 33.5%, and 48.3%, respectively [
128].
Furthermore, the thermodynamic and kinetic parameters of a PVC cable sheath were investigated by Liu et al. The range of activation energy in the first stage was 132–149 kJ/mol, with the average activation energy being 141 kJ/mol. On the other hand, the activation energy increased in the second stage to 193.8–266.4 kJ/mol, and the median activation energy was 235.3 kJ/mol using the Flynn–Wall–Ozawa method [
129]. Zhou et al. studied the upcycling of PVC waste into carbon compounds, chlorides, and pyrolysis gas using a one-pot dichlorination–carbonization-modification approach. The total solid yield of dechlorinated PVC was up to 80.8 wt% at 700 °C [
130].
Figure 5. Technology for recycling of PVC waste by dehydrochlorination and electrodialysis [
131].
Hydrothermal treatment is an effective method for removing chlorine from PVC [
125]. A hydrothermal supercritical water treatment was evaluated to depolymerize PVC waste into organic compounds, such as gas and liquid products. The chlorine atoms were dissolved in water and did not cause the formation of organochlorine compounds. The sequential occurrence of three reactions was then postulated as a mechanism, as follows: (1) the zipper dehydrochlorination method was used to remove HCl from PVC, causing conjugate double bonds to form in the polymer chain; (2) polymer chain breakage; (3) aromatic molecules were synthesized by combining broken chains [
132]. Enomoto et al. studied PVC depolymerization under high pressure in hot water [
133]. Hydrothermal depolymerization under supercritical and subcritical regions was investigated by Takeshita et al. After the 300 °C degradation process, the chlorine compound in PVC was dissolved in water, and hazardous chlorinated organic compounds were detected in the gas and liquid fractions. The primary product with a temperature reaction of 250–350 °C was polyene as a residual solid and an aliphatic–aromatic compound in the gas and liquid fractions [
132]. Zhao et al. studied the hydrothermal dichlorination of PVC wastes with an alkaline additive. Numerous chemical compounds were added, such as NH
3H
2O, KOH, Na
2CO
3, NaHCO
3, and NaOH, which took place in subcritical Ni
2+ and involved water at 220 °C for 30 min. In addition, Na
2CO
3 is the most promising additive, due to its high dichlorination efficiency up to 65.1% [
134].
5. Recycling of High-Density Polyethylene and Low-Density Polyethylene
High-density polyethylene (HDPE) and low-density polyethylene (LDPE) are the most popular forms of polyethylene. The manufacturing of HDPE adds the organometallic catalyst to polymerize ethylene. HDPE contains a higher proportion of crystalline regions than LDPE and is, hence, opaque and harder. The polymer chain in HDPE can be 500,000 to 1,000,000 carbon units long, with little branching. HDPE offers a wide range of applications in numerous plastic products, such as food containers, cleaning products, pipes, cables, tubes, and thin-film coating [
135]. The recycling of HDPE waste is conducted using fluid catalytic cracking, pyrolysis, and gasification; it follows the open-loop recycling of HDPE waste [
136].
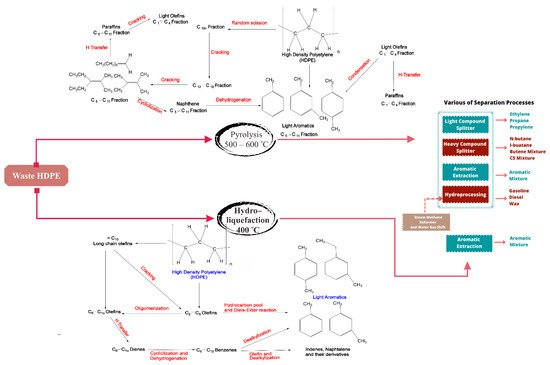
The possible routes of using depolymerized HDPE waste for aromatic hydrocarbon formation are shown in
Figure 6. The pyrolysis pattern of HDPE and LDPE is more intricate, and obtaining a large ethylene yield is challenging. The use of catalysts is an attractive solution to generate the desired product, especially with ethylene as the monomer of polyethylene. This process is called catalytic cracking. It can reduce energy consumption, due to lower temperatures than in thermal pyrolysis [
137,
138]. Singh studied the high yield liquid product of waste polyolefins pyrolysis, with a result of up to 92%, by applying MgCO
3 as a catalyst, with the primary products being aliphatic, alcohol hydrocarbons, ester, acetate, and aromatic [
139]. Furthermore, Zeolite, e.g., HZSM-5, is frequently utilized in major catalytic reactions, due to its larger specific area, high selectivity, pore structure, and acid group, which provides a hydrogen transfer reaction [
140,
141]. Integration of a fluidized bed reactor filled with HZSM-5 catalysts for the pyrolysis process, and pressure swing adsorption (PSA) for light components, as well as an inert gas, such as nitrogen separation, is one of the current technologies for polyethylene waste recycling process. Hernandez et al. found that the optimum temperature for FBR, and which produced high-yield gaseous compounds, was 500 °C [
142]. The presence of HZSM-5 favors C3–C5 hydrocarbons in large proportions. Meanwhile, Hernandez et al. investigated the HUSY catalyst with a lower ratio of silica/alumina and a larger surface area than the HZSM-5 catalyst. The results showed that C
5+ components were the primary product. The gaseous compounds from the pyrolysis reactor were condensed to split light and heavy components in the separator [
143].
Five distillation columns were used in light component separation to obtain ethylene, propane, and propylene. In the first distillation column, known as a demethanizer, methane was removed at 6 °C and 20 bar. The remaining light compounds were the bottom product of the demethanizer, and they then entered a second fractionation called a deethanizer, to split C
2 (ethane and ethylene) and C
3+ as the bottom product [
144]. Pure ethylene was obtained from the C
2 stream entering to deethylenizer, and a third distillation column was used with an operating temperature and pressure of −26 °C and 20 bar, respectively. The bottom product from the deethanizer was distilled in the fourth fractionation to produce C
3 (propane and propylene) and C
4+ products mixed with heavy components from the separator in the pyrolysis unit. The last distillation yielded high propylene purity in the top column and propane in the bottom column [
145].
Second step C
4 separation, is a technology used to recover n-butane, i-butane, butene mixture, and C
5 mixture. N-butane and i-butane are the raw materials for liquid petroleum gas (LPG) production, while butene mixture (trans-butene, 1-butene, isobutene, cis-2-butene, and 1,3-butadiene) and C
5 mixture (n-pentane and i-pentane) are frequently used as copolymers and solvents [
143,
146]. A mixture of heavy components from a light separation and pyrolysis unit was heated and split in a flash drum to remove the liquid aromatic mixture. The top product of the flash drum was condensed before entering the first fractionation, called the C
4 splitter. The column’s overhead liquid was sent to the n-butane separator (4 bar, 35 °C), where the n-butane product was placed in the bottom column. The liquid in the top n-butane column contained an i-butane and butene mixture, refined in the butene column (4 bar, 35 °C), whereas the overhead liquid was an i-butane stream at the bottom column, producing a butene mixture. Moreover, the bottom product of the C
4 splitter flowed, to the C
5 splitter to generate a C
5 mixture in the top section and the rest of the heavier hydrocarbon was in the bottom column, which was a mixed aromatic mixture stream from the flash drum [
147,
148].
Aromatic components contain a mixture of benzene, toluene, and xylene (BTX), compounds that are regularly utilized as a feedstock in chemical industries. Liquid–liquid extraction is a popular method for recovering aromatic mixtures. There are many types of solvents, such as triethylene glycol (TEG), sulfolane, N-formylmorpholine (NFM), and N -methyl-2-pyrrolidinone (NMP), utilized to extract aromatic compounds [
149,
150]. The sulfolane process patented by UOP is commonly used in commercial plants, due to its high selectivity and boiling point, but low dissolvability. Therefore, to solve these drawbacks, a mixture of two solvents is the best option to increase the selectivity and lower the recycling rate and ratio of extractant [
151,
152]. Extraction of aromatic compounds using a co-solvent of TEG and sulfolane was studied by Galie et al. The significant selectivity of xylene was increased.
Meanwhile, the solvent and recycle feed ratios were reduced by 20% [
151]. Other works showed that adding mixed solvents of sulfolane-NMP could extract 99% of benzene from reformate, and the distillate could be directly utilized as automobile gasoline [
153]. However, liquid extraction process units have some drawbacks, due to their high investment cost. Conventional distillation is widely used for purifying the mixture of the components. Nevertheless, due to binary azeotrope conditions, the aromatic mixtures cannot be separated by traditional distillation. Extractive distillation (ED) is an alternative method to extract aromatic hydrocarbons with a high energy efficiency, low equipment investment, and modest process units. ED requires a particular component to raise the volatility to near boiling point [
154,
155].
The solvent in liquid–liquid extraction can be applied as a third compound in ED. Wang et al. reported that co-solvents of NMP and sulfolane were used to extract aromatic components with the ED technique. A feed with aromatic and non-aromatic substances flowed to the ED column (2.5 bar, 120 °C). A non-aromatic product was fed to the rectifying column in the top column, to produce non-aromatic compounds, and solvents were carried over to the overhead ED column. The bottom stream of the ED column entered the solvent recovery and regeneration column (1.01 bar, 101.4 °C), to obtain a high purity of aromatic mixtures and regenerated solvents, to reuse in the ED column. The recovery of aromatics with the ED method reached 99.92% [
156].
Figure 6. Possible routes of using depolymerized HDPE waste for aromatic hydrocarbon formation (
top) by fast pyrolysis, using FCC spent catalysts in a fountain-confined conical spouted bed reactor, (
bottom) by hydro-liquefaction over Ni/HZSM-5 [
157,
158,
159].
On the other hand, adding hydrogen compounds is required to convert non-aromatic hydrocarbons into valuable products in a hydrogenator. Steam methane reforming, combined with a water gas shift reaction, is a mature process to produce hydrogen. A methane feed is mixed with steam, to carry out a methane reforming reaction. The products are hydrogen, carbon dioxide, carbon monoxide, and unconverted methane [
160]. In addition, the water gas shift process is needed to boost hydrogen levels. Then, a PSA is installed to purify hydrogen from carbon dioxide, carbon monoxide, and unconverted methane. Afterward, the high-purity hydrogen and non-aromatic hydrocarbons are first heated to 200–450 °C, before flowing into the hydrogenation reactor. Gasoline (C
6–C
7), diesel (C
8–C
16), and wax (>C
16) are produced in a hydrogenator using NiMo catalyst and can be applied as a fuel and chemicals [
161,
162].
Furthermore, Pan et al. investigated aromatic production from HDPE waste via the hydro-liquefaction method, using HZSM-5 with Ni to stabilize the aromatic product. Xylene was the dominant aromatic product, with a maximum yield of 28.9% at 400 °C. The aromatic selectivity reached 64.8%, with reaction time up to 4 h and a loading of Ni up to 15 wt% [
158]. The patterning process of hydro-liquefaction is typical of direct coal liquefaction. Therefore, constructing a hydro-liquefaction plant can implement a straightforward coal liquefaction process.
6. Recycling of Polyurethanes Waste
Polyurethanes (PUs) are essential materials, due to the thermoset and thermoplastic that can modify their chemical, thermal, and mechanical properties by reacting with polyisocyanates and polyols. The major polymers with urethane groups (–HN–COO–) are classified as PUs, regardless of the rest of the molecule [
170]. In addition, the polyether polyols based on polyethylene oxide, PP, aliphatic polyester polyols, tetrahydrofuran, polycarbonate polyols, aromatic polyester polyols, polybutadiene polyols, and acrylic polyols are the most often used polyols in the manufacturing of PUs [
171]. The thermochemical recycling of PUs includes alcoholysis, glycolysis, ammonolysis, and hydrolysis [
172].
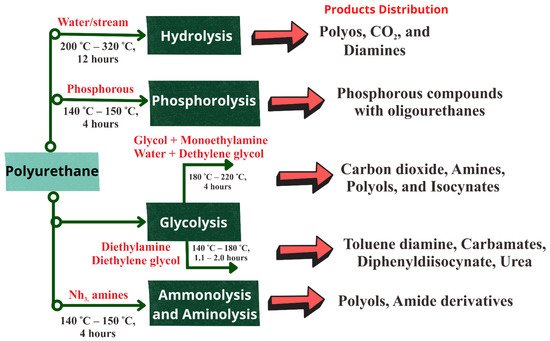
Figure 7 shows the alternative depolymerization routes for polyurethane waste.
Figure 7. Alternative depolymerization routes for polyurethane waste [
173].
Furthermore, numerous studies have considered recycling PUs by hydrolysis. The recycling products of PUs are a high-quality yield of polyol, isomeric toluene diamines, and CO
2. This was achieved by dry atmospheric pressure steam under a temperature range of 190–230 °C [
173]. Nevertheless, urethane linkages are quite stable, and protective groups such as the benzoxycarbony group are often utilized to cover their amino functions. As a result, PU hydrolysis must be performed using a strong acid, base, and quaternary compounds that can be added to generate the active hydrogen that contains polyethers and polyamines [
174]. The yield of toluenediamine vs. time shows the presence of a parallel first-order reaction equation, in which the urethane chain reacted up to 50 times quicker than urea. Urethane bonds were broken by direct hydrolysis, whereas urea bonds were broken via thermal fragmentation, to parent isocyanate and amine [
175]. The recycling of PU waste with 91% of I-PU and 98% of H-PU was hydrolyzed successfully by Motokucho et al. The operating conditions of this process, the CO
2 pressure and temperature of reaction, were up to 8.0 MPa and 190 °C, respectively. The water-soluble components were evaporated, to isolate the final products with a high yield [
176]. The major drawback of the hydrolysis process is that it consumes a lot of energy to heat the batch and provide high pressure in the reactor, resulting in an uneconomical process. As a result, hydrolysis has yet to be commercialized [
177,
178].
7. Recycling of Polyethylene Terephthalate
The chemical recycling of PET was reviewed, such as pyrolysis, hydrolysis, methanolysis, glycolysis, ionic liquid, phase-transfer catalysis, and combinations of glycolysis and hydrolysis, glycolysis and methanolysis, and methanolysis and hydrolysis in our previous study [
20]. Furthermore, reaction kinetics and conditions were investigated theoretically and experimentally. The recycling of PET is used to solve environmental problems and find another source of raw materials for petrochemical products and energy [
20]. The hydrocracking of waste plastic was pyrolyzed into high-quality liquid fuel using various catalysts, e.g., zeolite [
188]. On the other hand, PET can be reprocessed by mechanical recycling to develop wooden construction bricks for building purposes, with a composition of 75 wt% wood fiber and 25 wt% plastic waste, with a total hardness up to 21.270 HRR [
189]. Therefore, the mechanical recycling of plastics can be applied in large-scale industries, to solve environmental issues [
190].
This entry is adapted from the peer-reviewed paper 10.3390/polym14153133