Chitin and its derivative chitosan are highly abundant polymers in nature, appearing in both the shells and exoskeletons of various marine and non-marine species. Since they possess favorable properties, such as biocompatibility, biodegradability, non-toxicity, and non-immunogenicity, they have gained recent attention due to their enormous potential biomedical applications. The polycationic surface of chitosan enables it to form hydrogenic and ionic bonds with drug molecules, which is one of its most useful properties. Because chitosan is biocompatible, it can therefore be used in drug delivery systems. The development of chitosan-based nanoparticles has also contributed to the significance of chitin as a drug delivery system that can deliver drugs topically. Furthermore, chitin can be used in cancer treatment as a vehicle for delivering cancer drugs to a specific site and has an antiproliferative effect by reducing the viability of cells. Finally, chitosan can be used as a wound dressing in order to promote the faster regeneration of skin epithelial cells and collagen production by fibroblasts.
- chitin
- chitosan
- wound healing
- drug delivery
1. Introduction
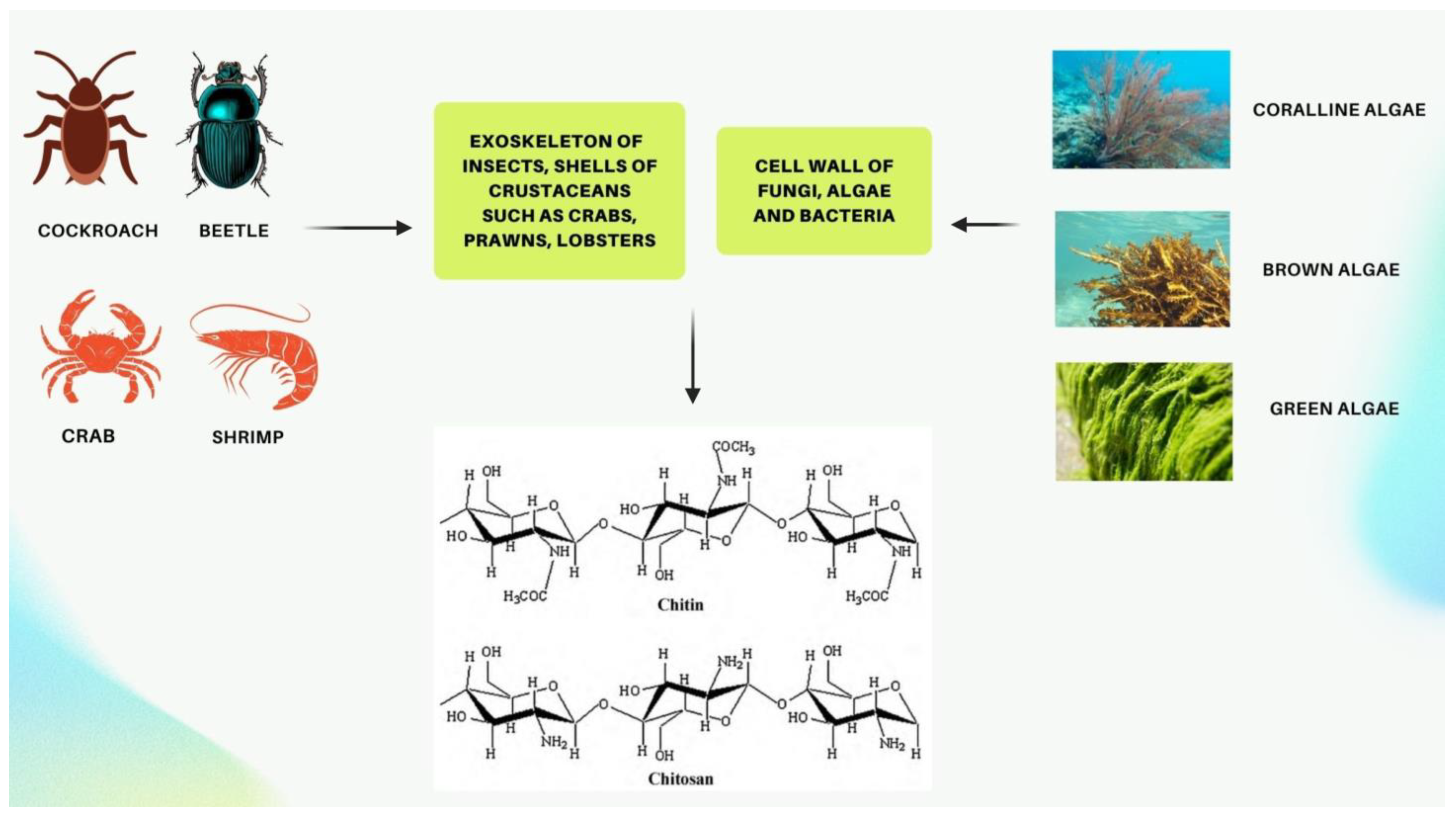
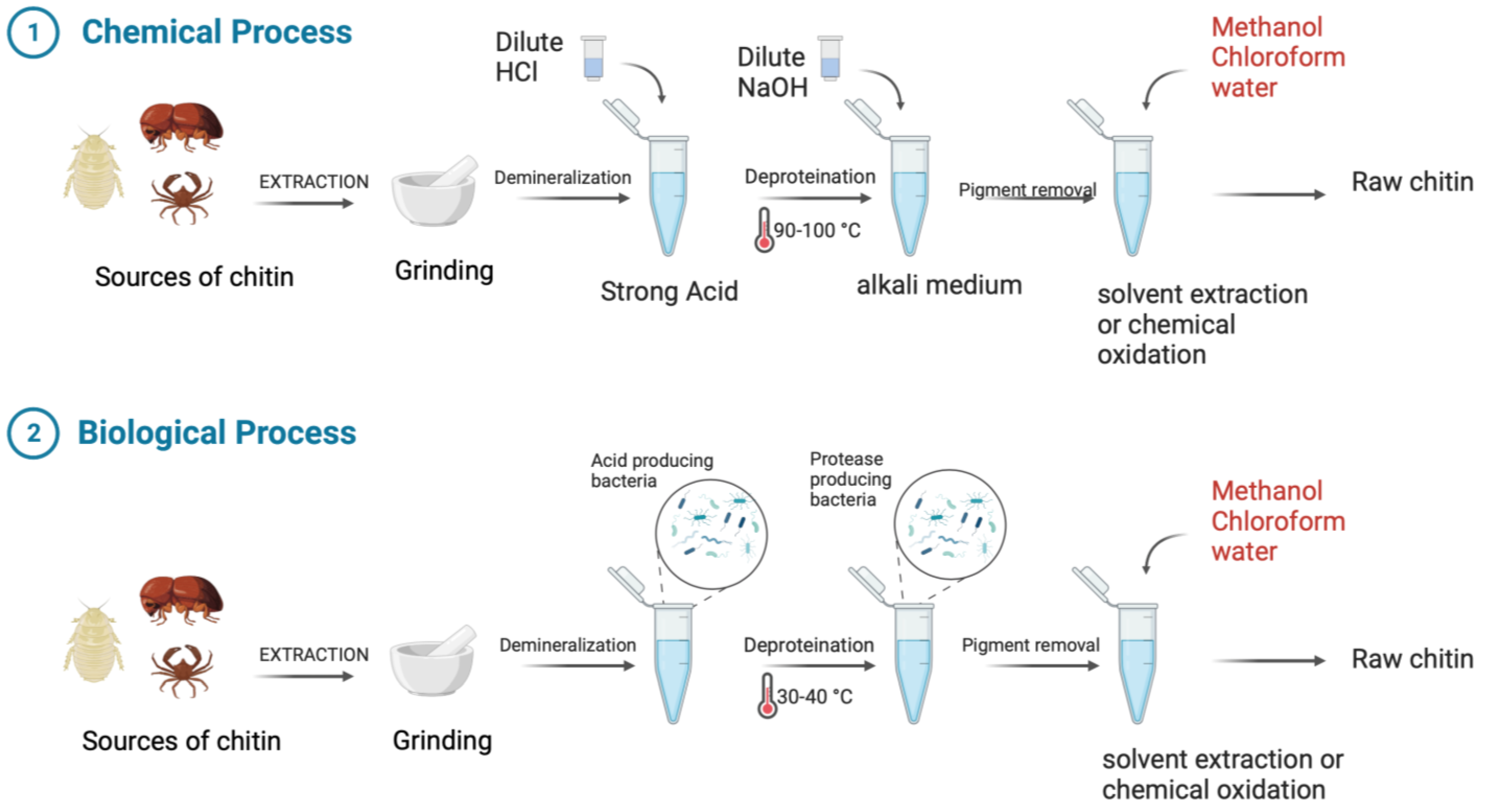
2. Chitosan as a Possible Drug Delivery Agent
This entry is adapted from the peer-reviewed paper 10.3390/md20070460
References
- Parhi, R. Chitin and Chitosan in Drug Delivery. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–239. ISBN 978-3-030-16581-9.
- Ahmad, A.; Mubarak, N.M.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent Advancement and Development of Chitin and Chitosan-Based Nanocomposite for Drug Delivery: Critical Approach to Clinical Research. Arab. J. Chem. 2020, 13, 8935–8964.
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173.
- Rahman, M.A.; Halfar, J. First Evidence of Chitin in Calcified Coralline Algae: New Insights into the Calcification Process of Clathromorphum Compactum. Sci. Rep. 2015, 4, 6162.
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The Role of Chitin-Rich Skeletal Organic Matrix on the Crystallization of Calcium Carbonate in the Crustose Coralline Alga Leptophytum Foecundum. Sci. Rep. 2019, 9, 11869.
- Ahmad, S.I.; Ahmad, R.; Shoeb Khan, M.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539.
- Singh, S.K. Solubility of Lignin and Chitin in Ionic Liquids and Their Biomedical Applications. Int. J. Biol. Macromol. 2019, 132, 265–277.
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic Liquids in the Processing and Chemical Modification of Chitin and Chitosan for Biomedical Applications. Green Chem. 2017, 19, 1208–1220.
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427.
- Broek, L.A.; Boeriu, C.G.; Stevens, C.V. Chitin and Chitosan: Properties and Applications; Wiley series in renewable resources; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-45046-7.
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.-W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330.
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036.
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc Chiton: Extraction, Characterization and Chitosan Preparation. Iran. J. Pharm. Res. IJPR 2017, 16, 366–379.
- Anraku, M.; Ifuku, S.; Iohara, D.; Hirayama, F.; Otagiri, M.; Gebicki, J.M. Future Aspects of Biomedical Applications of Chitin and Chitosan in Diseases Associated with Oxidative Stress. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 589–608. ISBN 978-0-12-817966-6.
- Tissera, W.M.J.C.; Rathnayake, S.I.; Abeyrathne, E.D.N.S.; Nam, K.-C. An Improved Extraction and Purification Method for Obtaining High-Quality Chitin and Chitosan from Blue Swimmer (Portunus Pelagicus) Crab Shell Waste. Food Sci. Biotechnol. 2021, 30, 1645–1655.
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866.
- Özel, N.; Elibol, M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel Biological and Chemical Methods of Chitin Extraction from Crustacean Waste Using Saline Water: Novel Biological and Chemical Methods of Chitin Extraction. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339.
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in Chitin Analytics. Carbohydr. Polym. 2021, 252, 117204.
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85.
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2012, 51, 12.
- Kaya, M.; Baran, T.; Karaarslan, M. A New Method for Fast Chitin Extraction from Shells of Crab, Crayfish and Shrimp. Nat. Prod. Res. 2015, 29, 1477–1480.
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider Chitin: An Ultrafast Microwave-Assisted Method for Chitin Isolation from Caribena Versicolor Spider Molt Cuticle. Molecules 2019, 24, 3736.
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594.
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally Drug-Loaded Chitin: Isolation and Applications. Mar. Drugs 2019, 17, 574.
- Zheng, J.; Lv, S.; Zhong, Y.; Jiang, X. Injectable Hydroxypropyl Chitin Hydrogels Embedded with Carboxymethyl Chitin Microspheres Prepared via a Solvent-Free Process for Drug Delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 1564–1583.
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 1–9.
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53.
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204.
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81.
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370.
- Shinde, U.A.; Joshi, P.N.; Jain, D.D.; Singh, K. Preparation and Evaluation of N-Trimethyl Chitosan Nanoparticles of Flurbiprofen for Ocular Delivery. Curr. Eye Res. 2019, 44, 575–582.
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008.
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309.
- Peterson, B.; Weyers, M.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Hamman, J.H. Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) That Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics 2019, 11, 33.
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019.
- Hasselbalch, H.C.; Holmström, M.O. Perspectives on Interferon-Alpha in the Treatment of Polycythemia Vera and Related Myeloproliferative Neoplasms: Minimal Residual Disease and Cure? Semin. Immunopathol. 2019, 41, 5–19.
- Lombardi, A.; Tsomos, E.; Hammerstad, S.S.; Tomer, Y. Interferon Alpha: The Key Trigger of Type 1 Diabetes. J. Autoimmun. 2018, 94, 7–15.
- Talpaz, M.; Mercer, J.; Hehlmann, R. The Interferon-Alpha Revival in CML. In Chronic Myeloid Leukemia; Hehlmann, R., Ed.; Hematologic Malignancies; Springer International Publishing: Cham, Switzerland, 2021; pp. 179–226. ISBN 978-3-030-71913-5.
- Pires de Mello, C.P.; Tao, X.; Kim, T.H.; Bulitta, J.B.; Rodriquez, J.L.; Pomeroy, J.J.; Brown, A.N. Zika Virus Replication Is Substantially Inhibited by Novel Favipiravir and Interferon Alpha Combination Regimens. Antimicrob. Agents Chemother. 2018, 62, e01983-17.
- Fleischmann, W.R.; Koren, S.; Fleischmann, C.M. Orally Administered Interferons Exert Their White Blood Cell Suppressive Effects via a Novel Mechanism. Proc. Soc. Exp. Biol. Med. 1992, 201, 200–207.
