TMD has multifactorial etiologies that affect the TMJ and the muscles of mastication, resulting in various symptoms, among which are pain, trismus, joint dislocation, clicking, and limited mouth opening. This may lead to a disability resulting in serious oral deficiencies, such as the emergence of oral mucositis, and affecting the oral health-related quality of life in 5 to 12% of the population [
3,
4]. Yet, the exact etiology of TMD is still unknown, and the most strategic conservative management of the condition remains debatable [
5,
6]. Hence, the treatment should be specific to the respective cause. A wide modality of treatments has been highly investigated, including the use of occlusal splints and/or pharmacotherapies, such as anesthetic, antidepressant, anticonvulsive, and non-steroidal anti-inflammatory drugs. However, long-term treatments lead patients to experience side effects because of adverse drug reactions. Therefore, non-pharmacological therapies, such as ultrasound, massage therapy, physiotherapy, acupuncture, exercise, transcutaneous electrical nerve stimulation, and photobiomodulation therapy (PBM-t) were proposed [
7].
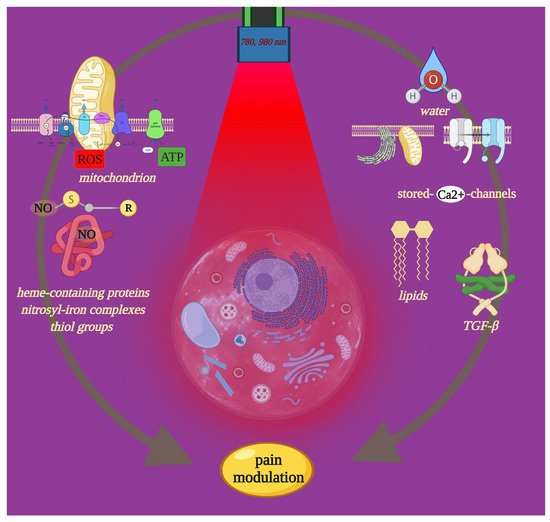
Furthermore, heme-containing proteins and nitrosyl-iron complexes can form complexes with nitric oxide molecules (NO) (i.e., NO-hemoglobin) and thiol groups (i.e., S-nitrosothiols). The ability of iron and sulfur to interact with light can therefore induce the release of NO from a variety of cellular sources [
16]. Plus, near-infrared light appears to excite water, affecting voltage-gated calcium (Ca
2+) [
17] channels and Ca
2+ stores [
18,
19] and lipids, which exhibit a mild but significant absorption peak in the range of 900–1000 nm [
20] (
Figure 1). Moreover, PBM is associated with significant neuropharmacological effects on the synthesis, release, and metabolism of neurochemicals in the cells, including serotonin, acetylcholine, histamine and prostaglandins, and glutamate [
21]. From a clinical point of view, it was seen that depending on the wavelength, type of target tissue, and tissue optical properties, the penetration depth of light energy used for PBM-t into human mucous varies considerably. The depth is maximal in the spectral range of near-infrared (~780–1000 nm), where the optical radiation penetrates to depths up to 4–6 mm [
22].
Figure 1. Near-infrared light can interact with molecular photoreceptor cellular involved in metabolism and homeostasis. The figure shows such light targets, which also play key roles in nociceptive signals. Through precise PBM-t, it is possible to modulate pain and support its management. Image created with BioRender.com.
2. Photobiomodulation in Management of Temporomandibular Joint Pain
2.1. Effect of PBM-t on Cell Pathways of Pain
The ability of PBM to modulate mitochondria and the mitochondrial dysfunction correlated to the etiology of pain were highly investigated. Mitochondria play an important role in a myriad of cell processes, including ATP production, biosynthetic pathways, oxygen sensing signaling, cellular redox homeostasis, ion homeostasis, and regulation of programmed cell death. As such, mitochondria modulation was suggested as an encouraging therapeutic strategy to prevent or mitigate chronic pain states [
36]. More precisely, the mitochondria’s vital role in cellular energy metabolism is long-known; mitochondria may generate more than 90% of the cell’s energy through ATP.
Literature evidence supports the role of ATP in pain mechanisms [
37]. Higher ATP levels are found in the articular fluid of arthritic knee joints, and endogenous ATP levels increase during inflammation [
38]. Thus, administration of ATP by iontophoresis in pain models increases the average pain evaluation in a dose-dependent way. Basically, the expression and the disruption of ATP receptors in sensory neurons are both involved in the increment and decrement of pain, respectively, in mice [
37].
In addition to ATP, reactive oxygen species (ROS) are by-products of mitochondria activities. In the physiological condition, they are usually removed by specialized cellular enzymes, such as superoxide dismutase, glutathione reductase, or catalase. Recent studies indicate that ROS play an important role in persistent pain [
39,
40]. ROS increment was observed in many pathophysiological conditions, including inflammation, where they may act as sensitizing on nociceptors [
41]. Removal of the high level of ROS by drugs produces analgesic effects in both neuropathic and inflammatory pain [
36,
41].
Therefore, PBM could support pain management thanks to the interaction of cytochrome with light as photoacceptors. For instance, isolated rat liver mitochondria in vitro irradiated by a low-power He-Ne laser experienced an increase in membrane potential, proton gradient, and ATP synthesis [
42]. Electron transfer and proton pumping activity are increased by laser stimulation as well [
43]. Recently, the effects of photobiomodulation on the redox state of healthy and cancer cells were described and the role of ROS elucidated [
44]. In addition, it was demonstrated that the 808 nm diode laser positively photobiomodulated the mitochondria oxygen consumption, the activity of the complexes III and IV, and ATP production [
12,
45,
46]. The 980 nm irradiation showed similar effects as well [
14]. However, the latter wavelength worked through window effects, and as a consequence, the mitochondria was stimulated, uncoupled, or not affected according to the therapy parameters used.
Abnormal neuronal Ca
2+ homeostasis, Ca
2+ channel expression, and function have been implicated in numerous diseases and common disorders such as pain [
47]. Voltage-gated calcium channels belonging to transient receptor potential (TRP) channels cellular sensors are mediators of pain signals in primary afferent neurons [
48]. Additionally, changes in Ca
2+ concentration may contribute to cell’s acidosis, which may be responsible for the enduring pain changes in nociceptor sensitivity. Hence, the calcium issue and the implication of voltage-gated calcium channels continue to be major areas of focus in the development of novel therapeutic approaches for pain treatment. Moreover, mitochondria play a key role in Ca
2+ intracellular homeostasis and affects membrane excitotoxicity. Wang et al. [
40] concluded that 980 nm affected temperature-gated calcium ion channels through intracellular water’s role as a photoacceptor. Amaroli and collaborators also showed the ability of 808 and 980 nm diode laser light to release intracellular stored calcium [
19,
49]. Notably, the Ca
2+ release induced the NO production through a like-neuronal NO synthase. The networking among Ca
2+, mitochondrion, ROS, ATP, and nitric oxide in PBM was highly investigated to point out the ability of light to modulate cellular fate [
18]. In addition, Colombo et al. reviewed the ability of PBM to affect NO homeostasis, leading to endothelial dysfunction recovery [
16]. Indeed, neuronal NO synthase activity is primordial in nociception, and the modulation of its expression is rapidly correlated to pain [
50]. Inflammatory cytokines and NO are involved in the pathogenesis of persistent and exaggerated pain states [
51]. In particular, evidence suggests that TGF-β is a relevant mediator of nociception with protective effects against pain [
52].
PBM-t increases the release of the anti-inflammatory cytokines IL-1RA and IL-10 and concurrent reduction of the pro-inflammatory IL-1α, IL-1β, IL-6, and IL-17 in irradiated murine mesenchymal cells [
53]. The 808 nm PBM-t mechanism might involve TGF-β-mediated control of pro-inflammatory interleukins [
53]. Additionally, photoactivation of the latent TGF-ß1 isoform but not TGF-ß2 or TGF-ß3 has been investigated after irradiation with an 810 nm laser diode system; it occurred via a specific methionine (position 253 on TGF-ß1) [
23].
2.2. Influencing Pain Recovery through PBM-t
The main differences were related to the laser parameters according to the dose-dependency for the treatment and the irradiation time. All of the included studies used continuous wave mode with no thermal relaxation except Madani [32], which adopted the gated mode characterized by a thermal relaxation time (TRT). However, TRT is not an important factor in PBM therapy, as this treatment has no appreciable thermal effects in the irradiated area due to the low-power parameter values used [54]. The power values in the included studies ranged from 0.04 W [33,34], 0.07 W [28], 0.1 W [30,31], and 0.3 W [29] up to 0.6 W [35].
Moreover, the irradiation time was not consistent among the studies. Refs. [28,31,32,33] stated the total time irradiation for each session, while refs. [30,34,35] provided the irradiation time for each trigger point. Sancakli [29] talked about the irradiation time without clarifying whether the corresponding values were the total processing time or the time per trigger point. In studies [28,30,32,33,34,35], the laser light beam was applied in contact mode. Studies [29,31] followed a non-contact protocol with a tip-to-tissue distance of 2 mm [29], while ref. [31] lacked to give any measurement. Consequently, the dose of energy density varied among studies and ranged between the least applied dose of 3 J/cm2 [29] and the highest applied dose of 100 J/cm2 [28]. In addition, the included studies did not specify in detail whether the fluences stated in their studies represented the total amount of energy density that was delivered to all the treated areas or the dose amount applied for each trigger point. Therefore, all this may present a distorted picture of the effectiveness of the applied dose. It is well-established that PBM is dependent on the dose delivered to the treated area [54]. The dose itself is dependent on the amount of energy delivered to the treated area at a certain time and through various delivery systems. Different dosages lead to different cellular responses and subsequently different clinical outcomes [12]. In addition, “if the power doubled and the time is halved, then the same energy is delivered but a different biological response is often observed” [13]. For deeper components such as TMJ, both the parameters and the procedure therapy description become mandatory. Thus, it is crucial to understand how much energy density should be applied to the skin to obtain this range of 4–10 J/cm2 at the cellular level, where the main problem exists, and taking into consideration the dramatical attenuation of light photonic energy as it crosses tissue multiple divergent layers. The Beer–Lambert law usually defines such a relationship. Additionally, none of the included studies mentioned any details about the beam profile characteristics, vitiating the therapy reproducibility of the selected studies. Indeed, the amount of energy density delivered into the treated area is closely related to the beam profile. With a conventional laser handpiece, the spatial beam profile is inherently Gaussian, and generally, as the tip-to-tissue distance increases, the energy density decreases [8]. These variables appear to be the main challenge for the PBM researchers and are key factors to take into consideration in PBM studies in the precision medicine field [55].
Another crucial consideration is the use of an optical power meter, which is an instrument for the measurement of the optical power (the delivered energy per unit time) in a light beam as a laser beam. It is well-established that light loses it is energy over time, and this applies to laser light as well. Many problems can cause a loss in power, including dirty optics, electrical problems, and limited lifespan [
60].
The diversity and some missing operational laser parameters, as shown in
Table 1, reflect the inconsistency in delivering valid reliable accurate PBM protocol and doses. In addition, all these discrepancies in laser operational parameters, method of laser application, and other conditions would surely influence the reliability and the uniformity of the outcomes. This in turn would limit the widespread acceptance of the PBM therapy as an effective treatment in the management of painful conditions such as TMD pain. Note that TMJ problems fluctuate, with spontaneous remission of some acute symptoms. They are also self-limiting sometimes, and thus, they may improve naturally without any intervention in some cases [
5].