The cardioprotective effects of dietary nitrate from beetroot in healthy and hypertensive individuals are undeniable and irrefutable. Nitrate and nitrate-derived nitrite are precursors for nitric oxide synthesis, which promotes positive effects on cardiomyocytes and myocardial ischemia/reperfusion, improving endothelial function, reducing arterial stiffness and stimulating smooth muscle relaxation, consequently decreasing systolic and diastolic blood pressures.
- beetroot-food interventions
- nitric oxide
- betanin
- polyphenols
- antioxidant activity
- clinical trials
Topic review
Encyclopedia
Beetroot, a Remarkable Vegetable
Diego dos S. Baião 1, Davi V. T. da Silva 1, and Vania M. F. Paschoalin 1,*
1Instituto de Química, Universidade Federal do Rio de Janeiro, Avenida Athos da Silveira Ramos 149, 21941-909, Rio de Janeiro – RJ, Brazil. diegobaiao20@ufrj.br (DSB); davivieira@ufrj.br (DVTS) and paschv@iq.ufrj.br (VMFP)
* Corresponding author:
Vania M. F. Paschoalin
Phone: 55 21 3938 7362
Fax: 55 21 3938 7266
E-mail: paschv@iq.ufrj.br
Definition
The cardioprotective effects of dietary nitrate from beetroot in healthy and hypertensive individuals are undeniable and irrefutable. Nitrate and nitrate-derived nitrite are precursors for nitric oxide synthesis, which promotes positive effects on cardiomyocytes and myocardial ischemia/reperfusion, improving endothelial function, reducing arterial stiffness and stimulating smooth muscle relaxation, consequently decreasing systolic and diastolic blood pressures. Moreover, beetroot is also enriched in other phytochemicals, such as betanin, saponins, polyphenols, and organic acids that may resist gastrointestinal digestion, raising the hypothesis that the cardioprotective effects of beetroot intake result from the combination of nitrate/nitrite and bioactive compounds that limit the generation of reactive oxygen species and downregulate pro-inflammatory genes while upregulating cytoprotective ones. Nitrate and phytochemical concentrations can be adjusted in beet formulations to fulfill requirements for acute or long-term supplementations, enhancing patient adherence to beet intervention to optimizing the health-promoting effects.
1. introduction
Epidemiological studies have demonstrated that dietary nitrate (NO3-) from certain vegetables can provide a physiological substrate for nitric oxide (NO) production which, in turn, supports cardiovascular function, causes vasodilation, and decreases blood pressure [1,2,3,4]. In addition, systematic reviews and meta-analyses have demonstrated the potential health benefits of the dietary intake of secondary metabolites found in vegetables, such as polyphenols, in decreasing the risk of chronic and degenerative diseases [5,6,7]. Food matrices derived from vegetables contain a complex mixture of these compounds, at variable concentrations, which may not yet have been well characterized [1].
Red beetroot (Beta vulgaris L. species), a member of the Betoideae subfamily, within the Amaranthaceae/Chenopodiaceae alliance, is a source of bioactive compounds, including dietary NO3-, betanin, phenolic and other antioxidant compounds, as well as dietary fiber, minerals (potassium, sodium, iron, copper, magnesium, calcium, phosphorus, and zinc) and vitamins (retinol, ascorbic acid, and B-complex) [1,8].
The consumption of a regular serving portion of in natura or minimally processed beetroot cannot provide an NO3- concentration capable of triggering cardioprotective effects. The administration of beet-traditional formulations in large amounts in order to reach pharmacological NO3- concentrations reduces adherence to nutritional beetroot interventions [9,10,11].
2. Beetroot (Beta vulgaris L.) Formulations
Dietary NO3- supplementation from beetroot requires smart formulations, to provide convenient serving portions containing effective NO3- concentrations and bioactive compounds as a feasible alternative to the consumption of in natura vegetables.
The combination of concentrated fresh beetroot juice and beetroot chips in different amounts can adjust bioactive compound concentrations and result in attractive and convenient NO3--rich beetroot products. Several beetroot formulations have been formulated to provide convenient and attractive dietary NO3- sources with the aim of stimulating NO production and promoting beneficial-health effects, according to the group population to be supplemented [10,11,12,13].

Beetroot juice is the original formulation for dietary NO3- supplementation and has been applied as a prime for most of the novel formulations proposed in recent years, according to the aim of the clinical study and the volunteer population to be tested [1,10]. Fresh and concentrated beetroot juice is produced from beets in a food centrifuge processor without adding water. Another formulation, a beetroot gel, designed to supplement athletes with dietary NO3- during sports competitions, is prepared by mixing concentrated fresh beetroot juice, crashed beetroot chips and carboxymethyl cellulose at a 90:17:3 ratio. Recently, a novel formulation, a beetroot cereal bar, a snack food to be consumed between major meals, was designed for chronic dietary NO3- administration to individuals who present risk factors for developing cardiovascular diseases (1). These beetroot-cereal bars were produced from the compaction of concentrated fresh beetroot juice, crashed beet chips and cereals, such as oats, wheat, soybeans, corn, and rice into 60 g pieces serving portions, measuring 10 cm × 3 cm × 1.5 cm [9,14,15].
3. Nutritional Composition of Beetroot Formulations
Beetroot-cereal bars present the highest content of protein, lipids, dietary fibers, sugars (fructose, glucose, maltose, sucrose), NO3- and saponin compared to beetroot gel, chips, and juice. Traces of nitrite (NO2-) (<0.5 mmol/100 g) and low lipid concentrations < 1 mmol/100 g were observed. Beetroot chips present the highest carbohydrate content (and energy) when compared to other beetroot formulations (Table 1).
Beetroot-cereal bars present the highest organic acids content compared to chips, gel, and juice (Table 2). Citric, ascorbic, malic, fumaric, succinic, and oxalic acids have been identified in all beetroot-derivatives, whereas succinic acid and oxalic acid have been found only in beetroot-cereal bars, both derived from the cereals added during the bar formulation. Furthermore, the beetroot-cereal bars also present the highest total phenolic compound content and diversity of these compounds when compared to chips, gel, and juice. Nine phenolic compounds were identified and quantified in the beetroot-cereal bar, namely, 3,4-dihydroxybenzoic, caffeic, chlorogenic, ferulic, gallic, p-coumaric, rosmarinic, syringic and vanillic acids. Gallic acid was the most abundant phenolic compound found in this product (Table 2).
|
Table 1. Beetroot formulations designed to fulfill cardioprotective effects: Proximate composition, sugars, NO3−, NO2−, betanin, saponin, organic acids and phenolic compounds |
||||
|
Compound |
Beetroot Formulations (100 g of product) |
|||
|
Cereal Bar |
Gel |
Chips |
Juice |
|
|
Ashes (%) |
1.30 ± 0.06b |
2.01 ± 0.13a |
1.00 ± 0.05c |
0.80 ± 0.06d |
|
Moisture (%) |
12.90 ± 0.50b |
76.14 ± 0.48a |
4.66 ± 0.57c |
85.50 ± 0.50a |
|
Energy (kilocalorie) |
325.58 ± 2.5b |
148.50 ± 0.01c |
365.05 ± 2.10a |
94.90 ± 1.70d |
|
Carbohydrate (g) |
62.97 ± 0.97b |
42.62 ± 0.31c |
89.96 ± 0.52a |
22.67 ± 0.40d |
|
Protein (g) |
16.20 ± 0.39a |
3.02 ± 0.09b |
0.97 ± 0.01c |
0.70 ± 0.07c |
|
Lipids (g) |
0.97 ± 1.00a |
0.66 ± 0.01b |
0.14 ± 0.01c |
0.16 ± 0.01c |
|
Total dietary fibers (g) |
4.07 ± 0.14a |
3.71 ± 0.10c |
3.22 ± 0.63b |
0.91 ± 0.31d |
|
Total sugars (g) |
37.72 ± 0.70a |
14.90 ± 0.23c |
18.79 ± 0.13b |
12.11 ± 0.35d |
|
Fructose (g) |
2.79 ± 0.15a |
1.21 ± 0.15b |
1.47 ± 0.11b |
0.86 ± 0.19c |
|
Glucose (g) |
4.71 ± 0.16a |
2.61 ± 0.12b |
2.70 ± 0.11b |
2.45 ± 0.21 b |
|
Sucrose (g) |
26.59 ± 0.80a |
11.60 ± 0.13c |
14.62 ± 0.17b |
8.80 ± 0.65 |
|
Maltose (g) |
3.63 ± 0.19a |
- |
- |
- |
|
NO3− (mmol) |
14.00 ± 0.05a |
6.30 ± 0.01b |
6.90 ± 0.02b |
4.10 ± 0.01c |
|
NO2− (mmol) |
0.20 ± 0.01a |
0.11 ± 0.02b |
0.13 ± 0.02b |
0.10 ± 0.02b |
|
Betanin (g) |
17.31 ± 0.12c |
24.65 ± 0.07b |
127.47 ± 0.01d |
30.1 ± 0.03a |
|
Saponins (mg) |
8648.00 ± 1.85a |
2200.00 ± 0.17d |
6371.00 ± 1.26b |
2599.00 ± 1.27c |
|
Organic acids |
||||
|
Citric acid (mg) |
231 ± 0.14a |
104 ± 0.10c |
152 ± 0.06b |
89 ± 0.10d |
|
Ascorbic acid (mg) |
155 ± 0.21a |
53 ± 0.04c |
93 ± 0.09b |
41 ± 0.03d |
|
Malic acid (mg) |
300 ± 0.10a |
159 ± 0.01c |
226 ± 0.10b |
134 ± 0.20d |
|
Fumaric acid (mg) |
81 ± 0.10a |
41 ± 0.20b |
63 ± 0.10a,b |
18 ± 0.10c |
|
Succinic acid (mg) |
51 ± 0.01 |
- |
- |
- |
|
Oxalic acid (mg) |
50 ± 0.15 |
- |
- |
- |
|
Total (mg) |
919 ± 0.71a |
357 ± 0.35c |
534 ± 0.35b |
284 ± 0.70d |
|
Phenolic compounds |
||||
|
Vanillic acid (mg) |
13.14 ± 0.11 |
- |
- |
- |
|
p-Coumaric acid (mg) |
39.68 ± 1.21 |
- |
- |
- |
|
Rosmarinic acid (mg) |
4.25 ± 0.04 |
- |
- |
- |
|
3,4-Dihydroxybenzoic acid (mg) |
9.97 ± 0.12a |
5.43 ± 0.81c |
7.85 ± 0.10b |
3.79 ± 0.03d |
|
Gallic acid (mg) |
60.50 ± 1.76a |
8.81 ± 0.15c |
22.49 ± 1.18b |
4.10 ± 0.06d |
|
Syringic acid (mg) |
4.48 ± 0.00a |
3.78 ± 0.02b |
4.47 ± 0.01a |
3.27 ± 0.05b |
|
Caffeic acid (mg) |
5.94 ± 0.03a |
3.34 ± 0.21b |
3.57 ± 0.06b |
2.90 ± 0.00c |
|
Ferulic acid (mg) |
3.23 ± 0.01a |
0.82 ± 0.11b |
0.88 ± 0.04b |
0.77 ± 0.01b |
|
Chlorogenic acid (mg) |
5.69 ± 0.01a |
3.27 ± 0.12b |
3.36 ± 0.02b |
3.17 ± 0.45b |
|
Total (mg) |
147.73 ± 3.30a |
25.45 ± 1.42c |
42.62 ± 1.39b |
18.00 ± 0.61d |
|
Values are expressed as means ± SD. Different letters within the same line indicate differences between samples at a significance level of p < 0.05. Beetroot-cereal bar and gel values are reproduced from Baião et al. [1] and da Silva et al. [12], respectively. Organic acids are expressed by 100 g of product |
||||
4. Beetroot Product Interventions Increase Nitric Oxide Production and Promote Health Benefits
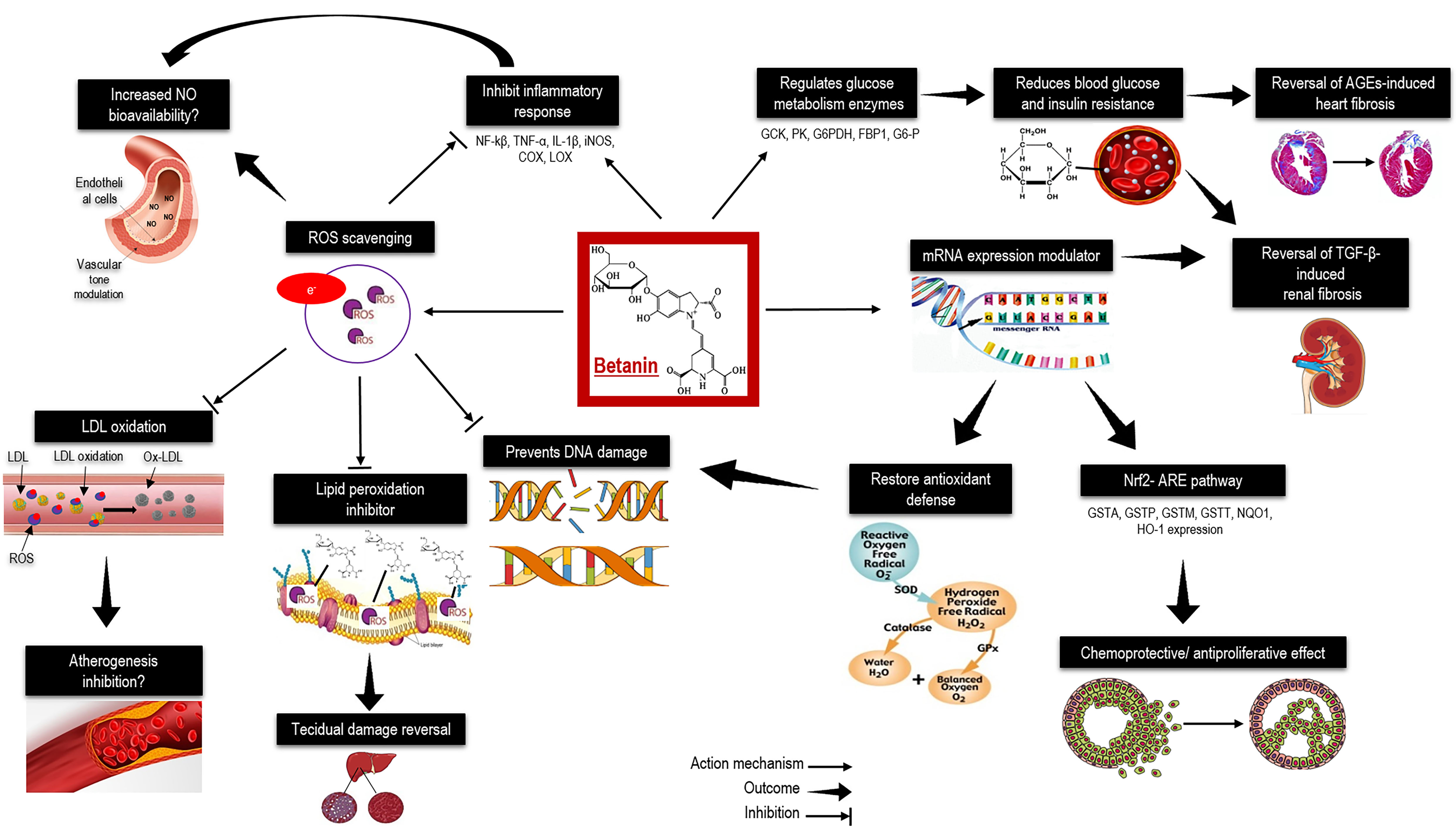
Betanin, a water-soluble pigment, that confers a red-violet colour to beetroot, is a powerful free radical scavenger due to its ability to donate electrons and hydrogen, which relies on the cyclic amine present in its structure, resembling ethoxyquin, a strong antioxidant, as well as hydroxyl groups (-OH), which are excellent hydrogen donors [16].
In vascular tissues, endothelial function is maintained and the atherogenesis process due to the antiradical activity of betanin (Figure 2). In addition, betanin can modulate redox-mediated signal transduction pathways involved in inflammation responses in endothelial cells by inhibiting the intercellular cell adhesion molecule-1 (ICAM-1), resulting in antiproliferative effects on human tumoral cells [17,18]. Betanin also regulates liver glucose metabolism-related enzymes in diabetes type II, such as those involved in the glycolytic pathways, like glucokinase, glucose-6-phosphatase, pyruvate kinase, in the pentose phosphate pathway, i.e., glucose-6-phosphate dehydrogenase, and in gluconeogenesis, like fructose-1,6-bisphosphatase [19].
Figure 2. Health effects of betanin: A summary of molecular and metabolic betanin targets reported in cell cultures and animal models. AGEs, advanced glycation end products; C, carbon; COX, cyclooxygenase; DNA, deoxyribonucleic acid; FBP1, fructose-bisphosphatase 1; G6-P, glucose 6-phosphate; G6PDH, glucose-6-phosphate dehydrogenase; GCK, glucokinase; GPx, glutathione peroxidase; GSTA, glutathione S-transferases A; GSTM, glutathione S-transferases M; GSTP, glutathione S-transferases P; GSTT, glutathione S-transferases T; H, hydrogen; H2O2, hydrogen peroxide; IL-1β, interleukin 1 beta; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; LDL, low-density lipoprotein; LOX; lipoxygenase; mRNA, messenger ribonucleic acid; N, nitrogen; NF-Κβ, nuclear factor kappa beta; NQO1, quinone dehydrogenase 1; NO, nitric oxide; Nrf2-ARE, nuclear factor erythroid 2-antioxidant responsive element; O, oxygen; O2•−, superoxide anion; OH, hydroxyl radical; Ox-LDL, oxidized low-density lipoprotein; PK, pyruvate kinase; ROS, reactive oxygen species; SOD, superoxide dismutase; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha.
Dietary NO3- is absorbed in the upper gastrointestinal tract, although 25% of ingested NO3- is captured by the salivary glands, where it is reduced to NO2- by commensal bacteria that express and secret the NO3--reductase enzyme in saliva. After the conversion of dietary NO3- to NO2- in the oral cavity, the NO2- in saliva is swallowed and reaches the stomach, where it is non-enzymatically decomposed in this acidic environment into NO and other bioactive nitrogen oxides by vitamin C or polyphenols [20].
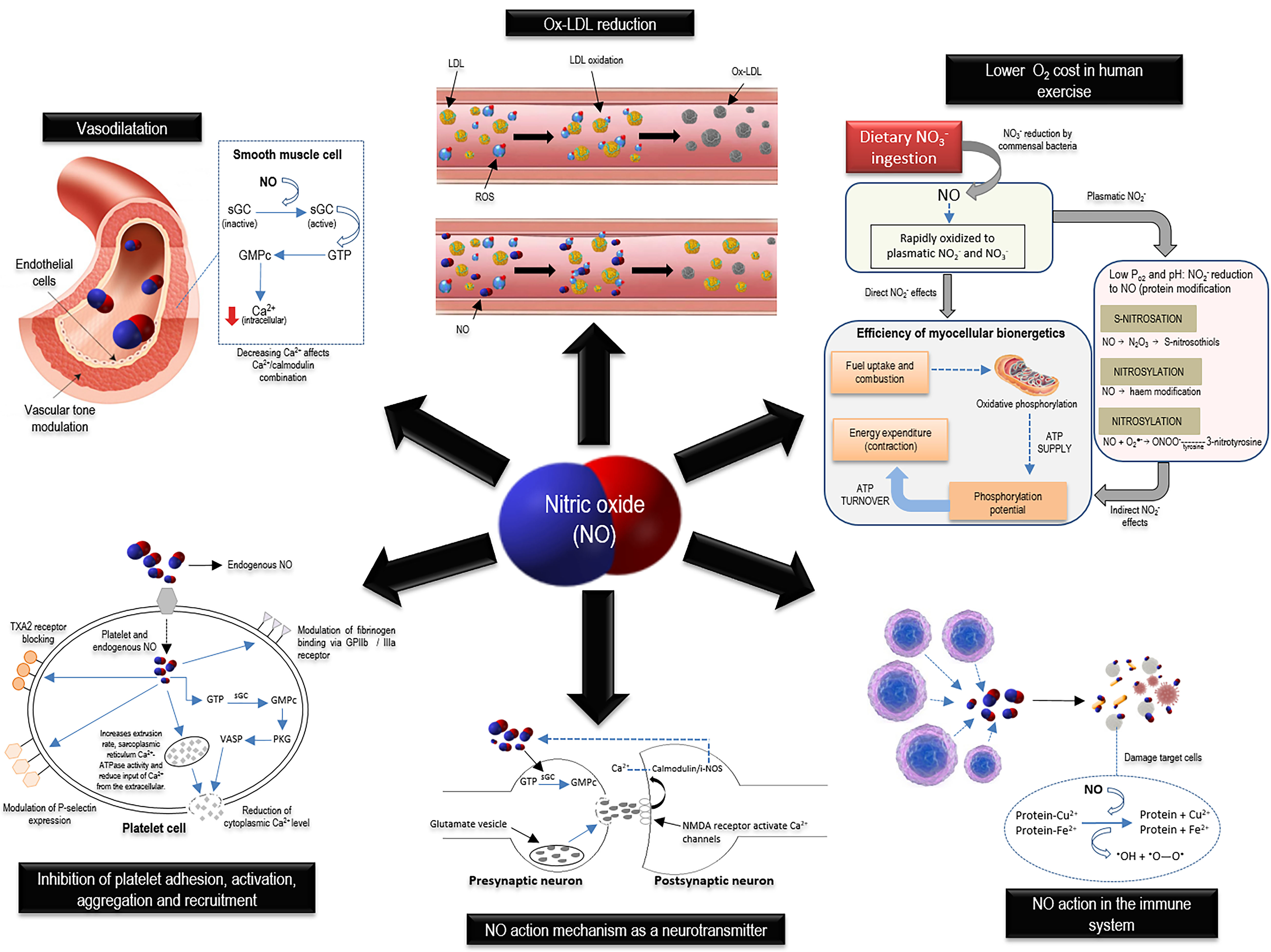
NO, a low molecular weight compound (30.01 g/mol) with a short life (from 5 to 10 s) produced in gas form, can exert antioxidant functions and is considered a secondary messenger in human physiology. It acts on the vascular endothelium, central and peripheral neurons, and the immune system, inhibiting platelet activation, adhesion, and aggregation, modulating vascular tone, and improving human skeletal muscle function [3,21,22,23]. NO promote these effects through multiple pathways, which depend on the cell tissue and the amount of produced NO (Figure 3).
Figure 3. Physiological role of nitric oxide in smooth muscle tissue vascular tone maintenance, synaptic transmission, cellular defense, hemostatic-thrombotic balance, and mitochondrial function. ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; Ca2+, calcium; Cu2+, copper; Fe2+, ferrous iron; GMPc, guanosine monophosphate cyclic; GPIIb, glycoprotein IIb; GPIIIa, glycoprotein IIIa; GTP, guanosine-5’-triphosphate; iNOS, inducible nitric oxide synthase; LDL, low-density lipoprotein; N2O3, dinitrogen trioxide; NMDA, N-methyl-D-aspartate; NO, nitric oxide; NO2-, nitrite; NO3-, nitrate; O2, oxygen; ONOO-, peroxynitrite; Ox-LDL, oxidized low-density lipoprotein; PKG, protein kinase G; PO2, pressure of oxygen; ROS, reactive oxygen species; sGC, soluble guanylate cyclase; TXA2, thromboxane A2; VASP, vasodilator-stimulated phosphoprotein.
Conclusions
Interventions with dietary NO3− from beetroot positively affect cardiovascular and metabolic functions by modulating the gene expression patterns or regulating the activity of proteins and enzymes involved in these cellular processes. The cytoprotective effects of NO-derived from the NO3--NO2-/NO pathway may be collectively reinforced by certain bioactive compounds naturally found in beetroot. Betanin seems to be another remarkable compound found in beetroot, and may be a putative candidate to attenuate the oxidative stress status in humans.
The entry is from https://doi.org/10.3390/antiox9100960
References
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]
This entry is adapted from the peer-reviewed paper 10.3390/antiox9100960
References
- Baião, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; Intech Open: London, UK, 2017; chapter 2, 21–44, doi:10.5772/intechopen.69301.
- Babateen, A.M.; Fornelli, G.; Donini, L.M.; Mathers, J.C.; Siervo, M. Assessment of dietary nitrate intake in humans: a systematic review. Am. J. Clin. Nutr. 2018, 108, 878–888, doi:10.1093/ajcn/nqy108.
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharm. 2013, 75, 677–696, doi:10.1111/j.1365-2125.2012.04420.x.
- Blekkenhorst, L.C.; Bondonno, N.P.; Liu, A.H.; Ward, N.C.; Prince, R.L.; Lewis, J.R.; Devine, A.; Croft, K.D.; Hodgson, J.M.; Bondonno, C.P. Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am. J. Clin. Nutr. 2018, 107, 504–522, doi:10.1093/ajcn/nqx046.
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18, doi:10.1016/j.jnutbio.2013.09.001.
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic deview and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316, doi:10.1093/aje/kww207.
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, E117, doi:10.3390/nu9020117.
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Drozdzal, K.; Witrowa-Rajchert, D.; Tylewicz, U. The Impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 2019, 8, 1–12, doi:10.3390/foods8070244.
- Baião, D.S.; Silva, F.O.; d`El-Rei, J.; Neves, M.F.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. A new functional beetroot formulation enhances adherence to nitrate supplementation and health outcomes in clinical practice. SDRP J. Food Sci. Technol. 2018, 3, 484–498, doi:10.25177/JFST.3.6.1.
- Baião, D.S.; Conte-Junior, C.A.; Paschoalin, V.M.F.; Alvares, T.S. Beetroot juice increase nitric oxide metabolites in both men and women regardless of body mass. Int. J. Food Sci. Nutr. 2016, 67, 40–46, doi:10.3109/09637486.2015.1121469.
- Da Silva, D.V.; Silva, F.O.; Perrone, D.; Pierucci, A.P.T.R.; Conte-Junior, C.A., Alvares, T.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Physicochemical, nutritional, and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nutr. Res. 2016, 60, 1–9, doi:doi.org/10.3402/fnr.v60.29909.
- Vasconcellos, J.; Conte-Junior, C.; Silva, D.; Pierucci, A.P.; Paschoalin, V.M.F.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. 2016, 25, 79–84, doi:10.1007/s10068-016-0011-0.
- Vasconcellos, J.; Silvestre, D.H.; Baião, D.S.; Werneck-de-Castro, J.P.; Alvares, T.S.; Paschoalin, V.M.F. A single dose of beetroot gel rich in nitrate does not improve performance but lowers blood glucose in physically active individuals. J. Nutr. Metab. 2017, 2017, 7853034, doi:10.1155/2017/7853034.
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379, doi:10.1016/j.foodchem.2009.09.016.
- Brazillian National Health Surveillance Agency. Technical Regulation on Complementary Nutrition Information; Resolution RDC n. 54.; Ministry of Health: Brasília, Brazil. 2012. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0054_12_11_2012.html (accessed on 13 May 2020).
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185, doi:10.1021/jf010456f.
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntiaficus-indica) inhibit endothelial ICAM-1 expression. Ann. N.Y. Acad. Sci. 2004, 1028, 481–486, doi:10.1196/annals.1322.057.
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Lida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer. Agents Med. Chem. 2011, 11, 280–284, doi:10.2174/187152011795347504.
- Dhananjayan, I.; Kathiroli, S.; Subramani, S.; Veerasamy, V. Ameliorating effect of betanin, a natural chromoalkaloid by modulating hepatic carbohydrate metabolic enzyme activities and glycogen content in streptozotocin - nicotinamide induced experimental rats. Biomed. Pharm. 2017, 88, 1069–1079, doi:10.1016/j.biopha.2017.01.146.
- Burleigh, M.; Liddle, L.; Muggeridge, D.J.; Monaghan, C.; Sculthorpe, N.; Butcher, J.; Henriquez, F.; Easton, C. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxide 2019, 89, 54–63, doi:10.1016/j.niox.2019.04.010.
- Wiczkowski, W.; Romaszko, E.; Szawara-Nowak, D.; Piskula, M.K. The impact of the matrix of red beet products and interindividual variability on betacyanins bioavailability in humans. Food Res. Int. 2018, 108, 530–538, doi:10.1016/j.foodres.2018.04.004.
- Vong, L.B.; Nagasaki, Y. Nitric oxide nano-delivery systems for cancer therapeutics: advances and challenges. Antioxidants 2020, 9, 1–14, doi:10.3390/antiox9090791.
- Richardson, G.; Hicks, S.L.; O’Byrne, S.; Frost, M.T.; Moore, K.; Benjamin, N.; McKnight, G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide 2002, 7, 24–29, doi:10.1016/S1089-8603(02)00010-1.