Patients with non-small cell lung cancer (NSCLC) develop bone metastasis (BoM) in more than 50% of cases during the course of the disease. This metastatic site can lead to the development of skeletal related events (SREs), such as severe pain, pathological fractures, spinal compression, and hypercalcemia, which reduce the patient’s quality of life. Recently, the treatment of advanced NSCLC has radically changed due to the advent of immunotherapy. Immune checkpoint inhibitors (ICI) alone or in combination with chemotherapy have become the main therapeutic strategy for advanced or metastatic NSCLC without driver gene mutations. Since survival has increased, it has become even more important to treat bone metastasis to prevent SRE. The lower efficacy of immunotherapy treatments in BoM+ patients could be induced by the presence of a particular immunosuppressive tumor and bone microenvironment.
1. Introduction
Lung cancer is the leading cause of cancer-related death [
1]. In recent years, there has been an improvement in cancer biology and immune system knowledge. In particular, the treatment of non-small cell lung cancer (NSCLC) has radically changed due to the introduction of new molecules with a molecular target and the advent of immunotherapy. Immune checkpoint inhibitors (ICIs), which target programmed-death 1 (PD1) and PD-ligand (PD-L1), either used alone or in combination with chemotherapy have become the main therapeutic strategies for advanced or metastatic NSCLC without driver gene mutations [
2,
3]—since progression free survival (PFS) and overall survival (OS) has improved. Nivolumab, atezolizumab, and pembrolizumab are recommended options for patients who progress after platinum-doublet chemotherapy. The Food and Drug Administration (FDA) approved nivolumab for this indication in October 2015 and atezolizumab in October 2016. Pembrolizumab received FDA approval for this indication in October 2015 with a limitation for PD-L1 positive tumors (with the accompained diagnostic IHC 22C3 pharmaDX test).
The incidence of bone metastasis in NSCLC varies ranging from 20% to more than 60%. Thanks to the improvement of diagnostic techniques (e.g., PET-CT scan) associated with increased survival, the incidence of bone metastasis seems to be increased.
Evidence has been published that indicates that bone marrow also functions in regulating the immune system and trafficking immune cells (regulatory T cells, T cells, B cells, dendritic cells, natural killer T cells, myeloid-derived suppressor cells, and mesenchymal stem cells) [
11]. Bone marrow, therefore, can be considered an immune system regulator and could potentially influence the response to immunotherapy. This is the new concept of osteoimmuno-oncology (OIO), which refers to interactions between bone, immune, and tumor cells in the bone metastatic microenvironment [
12].
2. Bone Metastasis and Microenvironment
Bone has a special immune microenvironment that is different from that of other organs. Pre-clinical studies have shown that bone is a particularly immunocompromised area. Most of the immune cells in the bone marrow are unable to control the proliferation of cancer cells. This is due to the presence of numerous immature and inhibitory immune cells in the premetastatic niche [11,16]. Furthermore, inside the bone marrow, the proportion of T cells is less than 5% (in peripheral blood 45–75%) and natural killer cells represent only 1–2% of lymphocytes.
About 40% of non-cytotoxic immune cells are regulatory T cells (Tregs) [17]. Other important cells with immunosuppressive activity are present in bone, such as myeloid-derived suppressor cells (MDSCs), which inhibit CD4 + T cells, CD8 + T cells, and NK cells [18]. In short, bone can be considered an immuno-privileged niche for disseminated cancer cells.
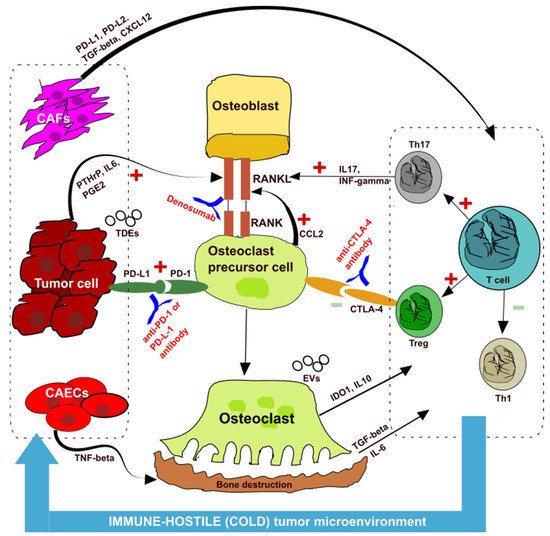
Tumor cells themselves release cytokines, which break the balance between osteoblasts and osteoclasts, thereby causing bone resorption and creating an immunosuppressive microenvironment—the so-called “vicious cycle” [
19,
21] (
Figure 1). Cancer cells, in fact, secrete parathyroid hormone-related peptide (PTHrP), prostaglandin E2 (PGE2), and other substances that promote the transformation of osteoblasts into pre-osteoclasts through the receptor-activator of nuclear kappaB ligand (RANKL) pathway, which then promotes differentiation into osteoclasts, causing bone destruction.
Figure 1. Interaction among the bone, immune system, and cancer cells. CAF: cancer-associated fibroblasts; CAECs: cancer associated endothelial cells; Th: T helper; Treg: regulatory T cells; PD-1: programmed-death 1; PD-L1: PD-ligand (PD-L1); CTLA4: cytotoxic T lymphocyte-associated protein 4; RANK: receptor-activator of nuclear kappaB; RANKL: receptor-activator of nuclear kappaB ligand; PTHrP: parathyroid hormone-related peptide; PGE2: prostaglandin E2; CCL2: chemokine (C-C motif) ligand 2; IDO–1: indoleamine 2,3-dioxygenase-1; IL: interleukin; TGF-beta: transforming growth factor-beta; IFN-gamma: interferon-gamma; TDE: tumor derived exosome; EVs: extracellular vesicles.
Tumors induce the release of chemokine (C-C motif) ligand 2 (CCI2) from pre-osteoclasts through the PD-1 pathway, which again promotes the formation of osteoclasts by RANKL pathway. So, hypothetically, the action of an anti-RANK antibody such as denosumab could act at this level to break the “vicious cycle”.
Osteoclasts secrete indoleamine 2,3-dioxygenase-1 (IDO1), interleukin 10 (IL 10), and other substances that induce immunosuppression (Figure 1). The release of transforming growth factor-beta (TGF-beta) due to bone resorption and interleukin 6 (IL6) create an immunosuppressive microenvironment as T cells differentiate into T helper 17 (Th17) and Treg instead of T helper 1 (Th1). This imbalance creates an immune-hostile (cold) tumor microenvironment (TME). Th17 lymphocytes secrete interleukin 17 (IL17) and interferon-gamma (INF-gamma), which again promote osteoclast differentiation.
An important role in this “vicious cycle” is also played by cancer-associated fibroblasts (CAF), which can induce an immunosuppressive microenvironment through PD-1 upregulation and TGF-beta secretion [
23] (
Figure 1).
Naturally, the RANKL-RANK-OPG (osteoprotegerin) pathway remains the central mechanism of osteolytic metastasis [
24,
25]. Since RANKL is also expressed in immuno-cells such as NK and T cells, the RANK/OPG balance regulates lymphocyte development in the lymph nodes, maintains dendritic cell activation, and regulates the mediated T response [
26]. Recently, the role of soluble RANKL has also been discovered. It can exert a chemotactic activity of the tumor cells in the bone without the involvement of the osteoclasts [
24].
In recent years, therefore, the RANKL pathway is considered the link between the bone and the immune system and could be considered a target for improving the efficacy of ICI therapy, as mentioned before [
16].
The news that has emerged in terms of the interaction between the tumor and the distant site of metastasis relates to the discovery of extracellular vesicles (EVs) [
27]. These carry important information that would induce the formation of a pre-metastatic bone niche.
Extracellular vesicles secreted by tumor cells and containing immunosuppressive molecules such as PD-L1 and TGF-beta can be immune escape mediators and may be a possible target for immunotherapy. We know that the PD-L1 secreted by tumor-derived exosomes (TDEs) suppress the activation of T cells in the lymph nodes and promote distant tumor proliferation [
28]. While anti-PD-L1 therapy is effective in reducing the immunosuppressive effect on PD-L1 expressing cells, the effect on PD-L1 of TDEs is poor, which may explain why an anti-PD1/PD-L1 therapy is ineffective in some tumors with high PD-L1 expression [
29,
30] (
Figure 1).
3. Bone Metastasis in NSCLC Treated with Immune Checkpoint Inhibitors
Table 1 summarized the results of papers on patients with bone metastasis treated with ICIs.
Table 1. The impact of ICI alone, in combination with chemotherapy (CT-ICI), or bone targeted therapy (BTT), according to different studies. ORR: overall response rate; PFS: progression free survival; OS: overall survival; ↑ increased; ↓ decreased; NV: not evaluable; NR: non reported; * only in patients treated with denosumab.
| |
Bone Metastasis (BoM)+ |
| Studies |
ICI Alone |
CT-ICI |
ICI
(Mono or Combo)
+BTT |
| |
ORR |
PFS |
OS |
ORR |
PFS |
OS |
ORR |
PFS |
OS |
| Liede A [35] |
NV |
NV |
NV |
NV |
NV |
NV |
↑ |
NR |
↑ |
| Zhu Y [36] |
NR |
↓ |
↓ |
NR |
↑ |
↑ |
NR |
↑ |
↑ |
| Li X [37] |
= |
↓ |
↓ |
= |
= |
= |
NR |
= |
= |
| Landi L [15] |
↓ |
↓ |
↓ |
NV |
NV |
NV |
NV |
NV |
NV |
| Qiang H [38] |
NV |
↓ |
↓ |
NV |
↑ |
↑ |
↑ |
↑ |
= |
| Bongiovanni A [39] |
↓ |
= |
↓ |
NV |
NV |
NV |
↑ |
↑ * |
↑ |
| Asano Y [40] |
↓ |
↓ |
↓ |
NV |
NV |
NV |
↑ |
NR |
↑ |
These data suggest that targeting the microenvironment to improve immunotherapy efficacy is a strategy that could be successful.
Several reported data, therefore, support the hypothesis that BTTs increase the activity of ICIs and reverse the negative impact of BoM. Of course, these data would be confirmed in prospective randomized clinical trials.
The clinical data of these studies could be explained through the “vicious cycle” theory and the presence of an immunosuppressive microenvironment at the bone and primary tumor level (Figure 1). In particular, the release of bone resorption cytokines, such as TGF-beta, increases Th17 suppressor lymphocytes and reduces Th1 effector lymphocytes, creating a cold microenvironment at the level of the primary tumor.
4. Conclusions
Bone is a special immune site with a unique immunosuppressive microenvironment. Bone metastasis impairs immunotherapy efficacy, especially when used alone. Even it is not true in all the trials, bone targeted therapies appear to have a synergistic effect when used in combination with ICIs—maybe due to the interruption of the “vicious cycle”. This action probably restores a less immunosuppressive (“cold” or “hostile”) tumor and bone microenvironment. These promising outcomes have to be confirmed in larger prospective trials—even better if randomized. In view of the new therapeutic scenario of NSCLC, further studies on the significance of extracellular vesicles and on new therapeutic approaches for bone metastasis are strongly recommended.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23126832