This paper gives an overview of different approaches to synthesize CNTs and then focuses on the floating catalyst method to form CNT sheets. A method is also described in this paper to modify the properties of macroscale carbon nanotube sheets produced by the floating catalyst method. This paper also discusses manufacturing obstacles and the possible commercial applications of the CNT sheet and CNTH sheet. Applications for CNT sheet include air and water filtering, energy storage applications, and compositing CNTH sheets to produce apparel with anti-microbial properties to protect the population from infectious diseases. The paper also provides an outlook towards large scale commercialization of CNT material.
- Carbon nanotubes
- hybrid sheet
- nanoparticles
1. Introduction
Over the last two decades, carbon nanotubes (CNT) have attracted attention in the fields of sensors [1], filtering [2,3], energy storage [4], and other areas. There are various methods to synthesize CNTs, including arc-discharge [5], using plasma [6], laser ablation [7], chemical vapor deposition (CVD) [8], and the floating catalyst method [9,10,11,12]. Arc-discharge and the laser ablation process are high-temperature synthesis processes which can produce CNTs with fewer structural defects. Many nanotubes with little control over chirality can be produced using the arc-discharge method but the use of metallic catalysts needed for the reaction requires post-processing purification of the nanotubes. Nanotubes synthesized using laser ablation show high yield and low metallic impurities because of the tendency of the metallic catalyst to evaporate from the end of the tube once it is closed. Nanotubes obtained from this process are not uniform and contain some branching. Furthermore, the use of a high purity graphite rod and laser power makes the method expensive. The arc-discharge, laser ablation, and plasma methods produce powder nanotubes. Lower temperature synthesis processes, such as chemical vapor deposition, are commonly used due to better control of nanotube diameter, purity, length, orientation, and economical mass production of carbon nanotubes [13]. High-quality single-walled nanotubes (SWNTs) were prepared with the CVD method using a novel aerogel-supported Fe/Mo catalyst with high productivity. The number of nanotubes obtained per unit weight of catalyst showed a five-fold improvement compared to catalyst supported on Al2O3 powder. The strong interaction between the aerogel and catalyst and the high surface area of the support resulted in high catalytic activity [14]. The CVD method with a substrate produces forests of CNTs that can be pulled as a thin film and wrapped layer by layer into sheet in a secondary operation. The floating catalyst gas-phase pyrolysis method can produce industrial-scale carbon nanotube sheet directly by winding a web or sock of nanotubes onto a drum in a continuous process. High-quality, evenly distributed, aligned carbon nanotubes of 10-nm diameter can be prepared by the floating catalyst method. In one approach, to enhance the growth of nanotubes, benzene was used as the carbon source, ferrocene as the catalyst, and thiophene as the sulfur-containing additive [15]. The relation between the average nanotube diameter and thiophene concentration was studied [16]. The amount of sulfur added can vary the morphology of the nanotubes. An improved floating catalyst approach allowed lower growth temperature and closer control over the growth parameters to produce large quantity, high-quality, and low-cost single-walled carbon nanotubes. This method can be used to grow both single-walled and multi-walled carbon nanotubes (MWCNTs) and the yield is affected by the growth promoter which is a sulfur-containing additive [16].
The unique properties of CNT materials include good current carrying capability [17,18] good mechanical strength [19], high thermal conductivity [20,21], and others. The anisotropic heat conduction of CNT sheet, for example, makes it a promising candidate for firefighting applications. A smart garment concept was shown in a simulation to direct heat from the garment to an external cold sink, which lowered the temperature of the body [22]. Fire retardant properties of free-standing CNT sheets were also investigated and considered appropriate to prepare personal protective equipment (PPE) for firefighters [23]. Overall, the integration of CNT materials into textiles opens a wide area of applications [24]. The porous structure of very thin CNT sheet, or sheet containing particles, and the electrical conduction properties of CNT sheet also make it a promising candidate for energy storage for wearable electronics [25] textiles.
The porous nature of thin CNT sheet or sheet with particles, and the high surface area of CNTs, may be useful for air and water filtering applications. The conductive and antimicrobial properties of CNTs can be used for pathogen/virus capture and inactivation. To enhance the antimicrobial property, anti-viral nanoparticles (e.g., Ag, Cu, ZnO, etc.) can be integrated into the CNT synthesis process [26]. Sheet performance depends on the customization of the sheet, not just the intrinsic properties of the CNT. Customization can include altering the hydrophilicity and breathability of the sheet and integrating different types of nanoparticles (NPs) into the synthesis process to change the properties of the sheet.
2. Characterization of CNT Material
CNT use in different applications has kept growing since its first discovery in 1991 by Iijima [35]. Applications include fiber-reinforced material [36], water filtration, air filtration, energy storage [37], supercapacitors [38], textiles [39], structural health monitoring, and hydrogen sensors [40], to mention a few. The increasing integration of CNT material in various applications can be accelerated by doping CNT material with different elements of the periodic table to make it multifunctional. Multifunctional CNT hybrid material possesses enhanced properties relative to pristine CNT material, and may outperform traditional material in the intended application. Pristine CNT can be doped with metals [41], polymers, ceramics, and various chemicals [38,42] to meet various application needs. As there is an increasing trend in making CNT hybrid materials, characterization of the material becomes an important factor. The lack of scientific and industrial standards to characterize novel CNT hybrid materials has accelerated the development of new measurement techniques and new parameters for characterizing CNT hybrid materials [43,44].
3. CNT for Energy Storage Applications
The discovery of carbon nanotubes has stimulated immerse interest among researchers in academia and industry for next-gen energy applications. Although many different CNT synthesis methods have been developed, the problems inherent in CNT synthesis have become more prominent along the way, such as very limited batch-process throughput, low purity, and low production yield. Floating catalyst CVD (FCCVD) has the potential to reduce these problems. Additionally, due to the flexible reactor design and unique continuous FCCVD process, the porous, flexible, freestanding films as-synthesized possess many advantages, which include good electrical properties, high mechanical reliability, and ease of handling. As an example, the CNT sheet has a large surface area and can be used to form the electrodes of a compact supercapacitor (Figure 8).
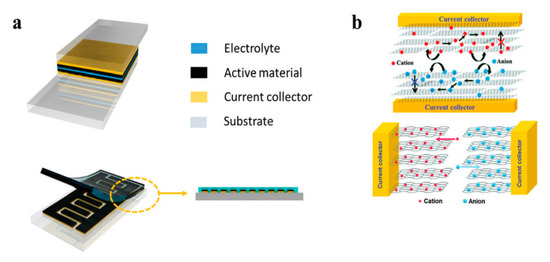
Charge storage has three mechanisms: the first involves an electrical double layer (EDL) capacitance, which stores charge on the surface of the electrode reversibly. The second is called the Nernstian process, also called a non-capacitive Faradaic process, which mainly follows the Nernst equation to describe the transfer of the localized valance electrons. The third is called the capacitive Faradaic process, which involves a pseudo-capacitor, the capacitive pathway based on the transfer of the delocalized valence electrons [47].
Transition metals and conductive polymers have been widely used as pseudocapacitive materials, which can provide capacitive Faradaic charge storage from the transfer of delocalized valence electrons [47]. Electrically conductive polymers (ECPs) are called synthetic metals due to their intrinsic electrical conductivity, which results from the full delocalization of π electrons on the long-chain aromatic polymer backbone [48]. Three major conducting polymers for active materials, i.e., polyaniline (PANI), poly (3,4-ethylene dioxythiophene) (PEDOT), and polypyrrole (Ppy), have demonstrated great potential in the application of energy storage owing to their high conductivity and superior capacitive properties. Conductive polymers paved the way for the exploration of CNT-conducting polymer composite materials. A supercapacitor device is illustrated in Figure 9.
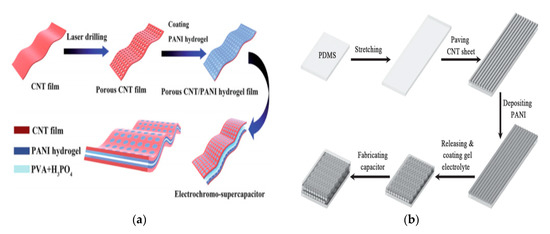
Several groups have used CNTs and related hybrids for supercapacitors and battery applications, either in sheet form or in fiber form. Although CNT-based energy storage shows encouraging results, one of the main challenges remaining is preventing the reduction in the surface area during the fabrication of the device. The reduced surface area has an adverse effect on the electrochemical performance of the electrodes, as well as providing limited space for loading active materials into the sheet, and it causes poor electrolyte diffusion [51].
4. CNT Filtering Applications
Carbon nanotube bundles are held together by van der Waals forces and these entangled nanotubes in a thin sheet create a porous structure which can be used to trap and immobilize virus/pathogens. Thick CNT sheets become almost impermeable. The inner core diameter of the carbon nanotube and nanotube growth can be controlled by the catalyst particle [52,53] and the core diameter is in the size range of many viruses, proteins, and other biological macromolecules [54]. The membrane can be formed by growing CNTs on a substrate and the voids must be treated with a filler material. Carbon nanotube membranes with a pore size of 2 nm were grown on a silicon chip. To produce a void-free membrane, spaces between the CNTs were sealed with silicon nitride. These membranes transported water and gas extremely fast which was supported by molecular dynamic simulations [55]. Vertically aligned carbon nanotube (VA-CNT) membranes showed an advantage over CNT membranes with filler material by eliminating the spaces between CNTs grown by CVD. No filler materials were used because interstitial pores were in the range of CNT pore size, hence more permeation area was available and resulted in higher porosity (~20%) [56]. Purification steps, such as heat treatment and acid treatment, remove the amorphous carbon and introduce functional groups which will improve the interaction of CNTs with other polymer and solvents. Chemical functionalization at the entry of CNT cores offers a nanoscale scaffolding to allow “gatekeeper” chemical interactions which improve both flux and selectivity of the separation application [57]. The porosity of CNTs can be improved by oxidation to attach functional groups such as COOH and OH. A large amount of oxygen-containing functional groups present at the surface of oxidized multiwall CNTs (MWCNTs) makes nanotubes more easily dispersed in the water and provides binding sites to interact with metal ions, which can be completely and easily adsorbed [58]. The interaction of iron with oxidized CNTs was found to be higher compared to raw CNTs because of the reduced diameter and fewer impurities due to the presence of an oxygen-containing group [59]. Modified MWCNT treated by a mixture of HCL and H2O2 and nitric acid showed 97% filtering efficiency for chromium. The removal efficiency of the modified filter was higher due to the large surface area and small nanotube diameter [60].
Carbon nanotubes have emerged as a promising solution for water purification due to their remarkably large surface area and their ability to be chemically functionalized to increase their affinity towards target molecules [61]. Magnetically aligned membranes showed a smoother surface and shearing along the axis of the applied magnetic field. The alignment was uniform across the surface and through the depth of the membranes [62]. A macroscopic hollow cylinder, designed by the spray pyrolysis method, successfully filtered heavy hydrocarbons from petroleum and bacteria from drinking water. The advantage of these kinds of filters over conventional filters is their reusability. The filters can regain their full filtering efficiency after applying a process such as ultrasonication and autoclaving [63]. Carbon nanotube membranes of sub-nanometer diameter provided efficient water desalination in reverse osmosis [64]. A CNT–metal filter developed by growing CNTs on the metal filter using the CVD method showed high filtration efficiency [65]. A plasma-modified ultra-long CNT (UCNT)-based porous membrane is used for the removal of salts, organic matter, and metal nanoparticles. Water treatment techniques, such as reverse osmosis, require high pressure and have limited mobility. UCNT-based membranes overcome these limitations as only a small amount of pressure is required to achieve sufficient water flux to desalinate water and remove organic and microbes using the free energy of adsorption for desalination. The adsorption capacity of these membranes was recovered by rinsing the membranes with tap water [66]. A multi-walled carbon nanotube/aromatic polyamide nanocomposite membrane rejected salts and humic acid by factors of 3.17 and 1.67, respectively, compared to the membranes without nanotubes. However, membrane permeability decreased by 6.5%, which can cause adsorptive fouling due to increased hydrophobicity and can degrade membrane performance in the long term [67]. A polyacrylonitrile (PAN) ultrafiltration membrane filled with hydroxyl-functionalized multi-walled carbon nanotubes improved the transport properties and the mechanical stability of the membrane. The inclusion of well-dispersed nanotubes increased the polymer solution viscosity and lowered the formation of macro voids [68]. A polymer-CNT composite membrane synthesized using interfacial polymerization was suitable for both aqueous solution-based nanofiltration and solvent resistant nanofiltration. Nanotubes were dispersed in an aqueous solution containing poly(ethyleneimine) (PEI) monomer, and the outer surface of the nanotubes was functionalized using hydrophobic and hydrophilic groups through microwave treatment. The base of the polymer membrane developed solute selectivity and a low resistance pathway at the interface and was created by the nanogaps at the outer surface of the nanotubes. The solvent fluxes were one order of magnitude higher than for commercial membranes [69]. Granular activated carbon (GAC) has a large surface area and a strong affinity for organic matter. The combination of a GAC filter as a pre-treatment followed by flocculation resulted in better removal of organics, a lowered flocculant dose, and helped in reducing biofouling of the membrane [70].
Volatile organic compounds (VOCs) present in the atmosphere lead to severe health issues. Carbon sorbents have high removal efficiency and are cost-effective. One study suggests that amorphous carbon provided more sorption capacity for organic chemicals and the curvature and topological defects affect the sorption process [71]. Pressure drop and filtration efficiency are two key factors of filter performance. A high pressure drop indicates high energy consumption which promotes high filter efficiency. There are a few mechanisms for particles to collide with the surface and deposit on the filter, such as diffusion, inertial impaction, and an interception. Inertial impaction and interception come into play when there is an increase in particle size. On the other hand, Brownian diffusion occurs when there is a decrease in particle size. In the case of intermediate particle size, no mechanism is dominant and two or more mechanisms occur simultaneously, which leads to maximum particle penetration through the filter, hence lower filter efficiency. Filters coated with a polytetrafluoroethylene (PTFE) membrane showed “v”-shaped efficiency curves for particle sizes from 10 to 300 nm at face velocities from 0.3 to 15 cm/s [72]. Nanofiber filters were synthesized by electrospinning nylon nanofibers on a microfiber substrate used in nano-aerosol filtration. It was found out that the flow was dominated by diffusion, considering that larger airflow reduces the retention time of the aerosol inside the network and hence lowers the time for the aerosol to deposit on the fibers. Due to the continuous loading of the submicron aerosol, the filter clogged and elevated the pressure drop. It is suggested that in high-efficiency filtration applications, stacking up low basis weight nanofiber layers to create a multi-layer filter is more effective than using a single high basis weight nanofiber layer filter [73]. In continuous loading of the aerosol on the filter, there will be less available pore space because formerly available free space is taken up by the aerosol, which results in lower permeability and higher pressure drop. This will create a serious problem in the upstream region. A “skin region’ will be formed at the upstream end of flow due to the packed dense layer of aerosols. A dual-layered arrangement with microfiber upstream and nanofiber downstream was proposed. The study showed that upstream microfibers filtered the incoming aerosol, which reduced the degree of loading in the downstream nanofibers. The microfiber layer has a higher dust holding capability, so the aerosol deposition does not induce extra pressure drop. Now, there will be a uniform incoming aerosol layer at the downstream nanofiber which reduces the skin region [74]. A novel filter was prepared by drawing aligned nanotube sheets onto the polypropylene melt-blown sheet and embedding CNT layers in a cross-ply structure. The three-layer CNT cross-ply filter achieved the highest quality factor and met the high-efficiency particulate air (HEPA) filter criterion [75].
The recent outbreak of COVID-19 prompted a global response to biological aerosol exposure. SWNTs presented strong antibacterial activity. Direct interaction of bacteria with high purity, narrow-diameter nanotubes caused severe damage, and cell inactivation [76]. A comparative study of the antibacterial capability of highly purified SWNTs and MWCNTs showed that SWNTs have stronger antibacterial activity, indicating that the diameter of CNTs is a key factor in the inactivation of bacteria. A smaller-diameter nanotube can ease the penetration of nanotubes in the cell wall. Other factors that attributed to this increase were a large contact surface area and unique electrical and chemical properties [77]. A combination of nisin with a CNT-coated filter provided efficient bacterial capture and inactivation in one filtration step. Nisin adsorption on MWCNTs decreased the hydrophobicity of the surface, hence the greater the nisin adsorption, the lower the hydrophobicity [78]. The SWNT filter showed strong inactivation of bacterial and fungal aerosols in both indoor and outdoor environments. Filters with high CNT loading lowered both bacterial and fungal aerosol concentrations [79]. An electrochemical MWCNT microfilter showed removal and inactivation of the virus and bacterial pathogens at a low voltage of 1–3 V. The advantages over conventional filters include the antimicrobial activity of MWCNTs, high surface area, and small pore size which can sieve bacteria and remove bacteria by depth filtration, availability of large electrochemically active sites, and increased corrosion stability [80]. A CNT-metal oxide membrane composed of CNTs and silver nanoparticles improved the anti-bacterial property of the membrane. Silver acted as a welding agent to bond the CNTs together and transformed the membrane from hydrophobic to hydrophilic. The porosity of the membrane was also increased by increasing the silver content [81]. A novel CNT-based facepiece respirator was tested on a manikin-based system. Filters with high CNT loading improved the filtering performance and it was observed that CNT filters have higher biological aerosol particle filtration efficiency than total aerosol particles [82].
This entry is adapted from the peer-reviewed paper 10.3390/nano10102023
