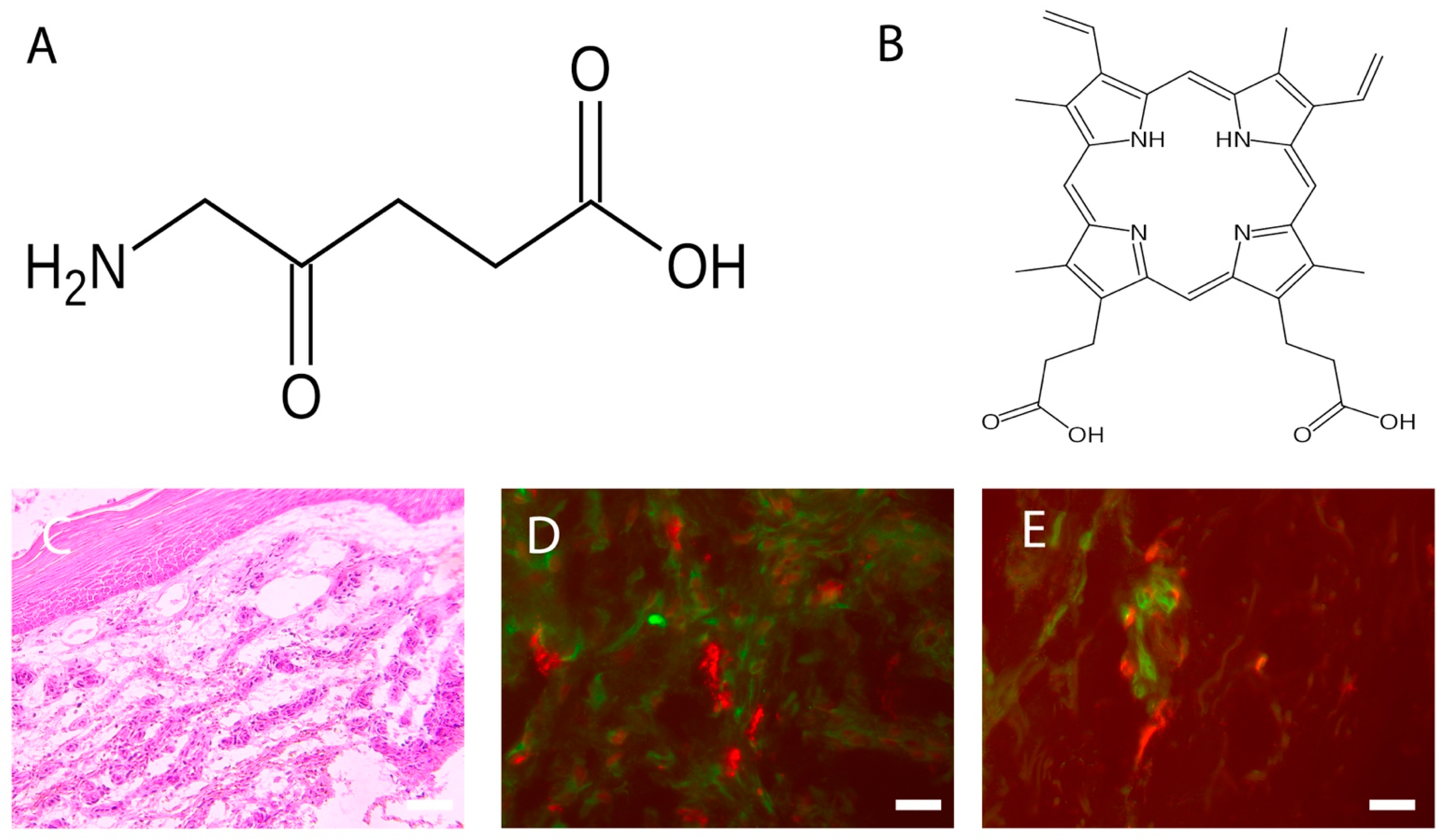
Photodynamic therapy (PDT) is a two-stage treatment that combines light energy with a photosensitizer designed to destroy cancerous and precancerous cells after light activation. Photosensitizers are activated by a specific wavelength of light energy, usually from a laser. The photosensitizer is nontoxic until it is activated by light. However, after light activation, the photosensitizer becomes toxic to the targeted tissue. Among sensitizers, the topical use of 5-aminolevulinic acid (ALA), a natural precursor of protoporphyrin IX, a precursor of the heme group, and a powerful photosensitizing agent, represents a turning point for PDT in the dermatological field, as it easily absorbable by the skin. Wound healing requires a complex interaction and coordination of different cells and molecules. Any alteration in these highly coordinated events can lead to either delayed or excessive healing.
- acute wounds
- cellular infiltrate
- chronic wounds
- mast cells
- photodynamic therapy
- nerves
- neurons
- wound healing
1. The Photodynamic Therapy
2. Photosensitizers
| Substances | Target | References |
|---|---|---|
| HSP 47 (Antibody) | Fibroblasts | [10] |
| Avidin (Egg white protein linking biotin) | MCs | [11] |
| MHC class II (Antibody) | Dendritic cells | [12] |
3. Wound Healing
4. PDT and Wound Healing
5. PDT and Chronic Wounds
5.1. The Response of Cellular Infiltrate
5.2. Neuroimmunomodulation
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10071624
References
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An up-to-date review. Appl. Sci. 2021, 11, 3626.
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—mechanisms, photosensitizers and combinations. Biomed. Pharm. 2018, 106, 1098–1107.
- Grandi, V.; Sessa, M.; Pisano, L.; Rossi, R.; Galvan, A.; Gattai, R.; Mori, M.; Tiradritti, L.; Bacci, S.; Zuccati, G.; et al. Photodynamic therapy with topical photosensitizers in mucosal and semimucosal areas: Review from a dermatologic perspective. Photodiagnosis Photodyn. Ther. 2018, 23, 119–131.
- Tampa, M.; Sarbu, M.; Matei, C.; Mitran, C.; Mitran, M.; Caruntu, C.; Georgescu, S. Photodynamic therapy: A hot topic in dermato-oncology. Oncol. Lett. 2019, 17, 4085–4093.
- Donnelly, R.F.; McCarron, P.A.; Woolfson, A.D. Derivatives of 5-aminolevulinic acid for photodynamic therapy. Perspect. Med. Chem. 2007, 1, 49–63.
- Wang, B.C.; Fu, C.; Qin, L.; Zeng, X.Y.; Liu, Q. Photodynamic therapy with methyl-5-aminolevulinate for basal cell carcinoma: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2020, 29, 101667–101679.
- Tedesco, A.; Jesus, P. Low level energy photodynamic therapy for skin processes and regeneration. In Photomedicine. Advances in Clinical Practice; Yohey, T., Ed.; Intech Open: London, UK, 2017.
- Lecci, P.P.; Corsi, A.; Cappugi, P.P.; Bacci, S. La terapia fotodinamica nel trattamento delle lesioni cutanee croniche. In Evidenze Cliniche e Pratica Sperimentale; Aracne Editrice: Rome, Italy, 2013; pp. 1–64.
- National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/reactive-oxygen-species (accessed on 28 June 2022).
- Goodpaster, T.; Legesse-Miller, A.; Hameed, M.R.; Aisner, S.C.; Randolph-Habecker, J.; Coller, H.A. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem. 2008, 56, 347–358.
- Bergstresser, P.R.; Tigelaar, R.E.; Tharp, M.D. Conjugated avidin identifies cutaneous rodent and human mast cells. J. Investig. Derm. 1984, 83, 214–218.
- ten Broeke, T.; Wubbolts, R.; Stoorvogel, W. MHC class II antigen presentation by dendritic cells regulated through endosomal sorting. Cold Spring Harb. Perspect. Biol. 2013, 5, a016873.
- Bacci, S.; Bani, D. The epidermis in microgravity and unloading conditions and their effects on wound healing. Front. Bioeng. Biotechnol. 2022, 10, 666434.
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Derm. 2015, 173, 370–378.
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620.
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94.
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflam 2019, 2019, 3706315–3706329.
- Visha, M.G.; Karunagaran, M. A review on wound healing. Int. J. Clin. Correl. 2019, 3, 50–59.
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735.
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223–200236.
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700.
- Douahiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252.
- Bacci, S. Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int. J. Mol. Sci. 2022, 23, 1820.
- Zhang, Z.; Kurashima, Y. Two sides of the coin: Mast cells as a key regulator of allergy and acute/chronic inflammation. Cells 2021, 10, 1615.
- Nesi-Reis, V.; Lera-Nonose, S.V.; Oyama, J.; Ramos-Milaré, Á.; Demarchi, I.; Alessi-Aristides, S.; Vieira-Teixeira, J.J.; Verzignassi Silveira, T.G.; Campana-Lonardoni, M.V. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2018, 30, 294–305.
- Oyama, J.; Ramos-Milaré, Á.; Lera-Nonose, S.V.; Nesi-Reis, V.; Demarchi, I.; Alessi-Aristides, S.; Vieira-Teixeira, J.J.; Verzignassi Silveira, T.G.; Campana-Lonardoni, M.V. Photodynamic therapy in wound healing in vivo, a systematic review. Photodiagnosis Photodyn. Ther. 2020, 10, 101682.
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11.
- Corsi, A.; Lecci, P.P.; Bacci, S.; Cappugi, P. Chronic wounds treated with photodynamic therapy: Analysis of cellular response and preliminary results. Acta Vulnol. 2013, 11, 23–33.
- Corsi, A.; Lecci, P.P.; Bacci, S.; Cappugi, P.; Pimpinelli, N. Early activation of fibroblasts during PDT treatment in leg ulcers. G Ital. Derm. Venereol. 2016, 151, 223–229.
- Grandi, V.; Bacci, S.; Corsi, A.; Sessa, M.; Puliti, E.; Murciano, N.; Scavone, F.; Cappugi, P.; Pimpinelli, N. ALA-PDT exerts beneficial effects on chronic venous ulcers by inducing changes in inflammatory microenvironment, especially through increased TGF-beta release: A pilot clinical and translational study. Photodiagnosis Photodyn. Ther. 2018, 21, 252–256.
- Harding, K.G.; Morris, H.L.; Patel, G.K. Healing chronic wounds. Br. Med. J. 2002, 324, 160–163.
- Toporcer, T.; Lakyová, L.; Radonak, J. Venous ulcer-present view on aetiology, diagnostics and therapy. Cas. Lek. Ceskych 2008, 147, 199–205.
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610.
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48.
- Kyaw, B.M.; Järbrink, K.; Martinengo, L.; Car, J.; Harding, K.; Schmidtchen, A. Need for improved definition of chronic wounds in clinical studies. Acta Derm. Venereol. 2018, 12, 157–158.
- Yang, T.; Tan, Y.; Zhang, W.; Yang, W.; Luo, J.; Chen, L.; Liu, H.; Yang, G.; Lei, X. Effects of ALA-PDT on the healing of mouse skin wounds infected with Pseudomonas aeruginosa and its related mechanisms. Front. Cell Dev. Biol. 2020, 8, 585132.
- Haensel, D.; Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev. Dyn 2018, 247, 473–480.
- Kushwah, R.; Hu, J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011, 1, 20.
- Murciano, N.; University of Florence, Florence, Italy. Personal communication, 2016.
- Steinmann, L. Elaborate interactions between the immune and nervous system. Nat. Immunol. 2004, 5, 575–581.
- Ashrafi, M.; Baguneid, M.; Bayat, A. The role of neuromediators and innervation in cutaneous wound healing. Acta Derm. Venereol. 2016, 96, 587–594.
- Laverdet, B.; Danigo, A.; Girard, D.; Magy, L.; Demiot, C.; Desmoulière, A. Skin innervation: Important roles during normal and pathological cutaneous repair. Histol. Histopathol. 2015, 30, 875–892.
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067.
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085.
- Siiskonen, H.; Harvima, I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front. Cell Neurosci. 2019, 13, 422.
- Grandi V, Paroli G, Puliti E, Bacci S, Pimpinelli N. Single ALA-PDT irradiation induces increase in mast cells degranulation and neuropeptide acute response in chronic venous ulcers: A pilot study. Photodiagnosis Photodyn Ther. 2021 Jun;34:102222. doi: 10.1016/j.pdpdt.2021.102222
