Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
There has been considerable interest in icariin (ICA) and its derivates, icariside II (ICS) and icaritin (ICT), due to their wide range of potential applications in preventing cancer, cardiovascular disease, osteoporosis, delaying the effects of Alzheimer’s disease, treating erectile dysfunction, etc.
- bioavailability
- icariin
- icariside II
- icaritin
1. Introduction
The most plentiful polyphenols occurring in food are the natural flavonoids and their glycosides. To date, more than 15,000 different flavonoids have been identified and isolated from plants [1]. Recently, due to their various health-promoting properties [2], an abundance of food supplements containing those compounds have gained popularity among the general population [3].
Icariin (ICA), icariside II (ICS), and icaritin (ICT) are the main active components of Epimedii, a traditional Chinese medicine that was used to treat and prevent numerous health disorders, such as cardiovascular diseases, osteoporosis, or sexual dysfunction [4]. Recently, several studies investigated their potential as drugs against many common health issues. In some cases, their medicinal effects were greater than those of certain currently used drugs [5][6]. For example, in the case of Alzheimer’s disease prevention, they have approximately the same efficacy as donepezil, but they are less toxic [7].
Among various other factors, the low bioavailability of flavonoids remains a major obstacle to increasing their effectiveness [8]. As with other flavonoids, unfortunately, icariin and its derivates also have a low oral bioavailability (~12%) and poor absorption; thus, their clinical applications are limited [9]. Their poor oral bioavailability is caused by low water solubility and membrane permeability, a slow dissolution rate in biological fluids, etc. Compared to other routes, oral administration assures greater comfort, less pain, and, most importantly, higher patient compliance [10]. Therefore, more attention should be paid to overcoming this hurdle.
2. Icariin and Its Derivates
In ancient China and thence, elsewhere, the herbal medicine Herba Epimedii has been used as a tonic due to its powerful anti-rheumatic properties and aphrodisiac effects [11]. According to the Chinese pharmacopeia, the main active component of the Epimedium sp. was found to be icariin and icariside II, the last being also called baohouside-I [12]. With mass spectrometry methods, the epimedins A–C and sagittatoside B have been established as other active components of the Herba Epimedii [13]. Several metabolism studies performed by means of HPLC methods have found that the intestinal flora metabolized the icariin, converting it mainly into icariside II, icaritin, and desmethylicaritin [14]. It was demonstrated that ICS is the secondary metabolite because its content exceeded every other active agent [15].
2.1. Extraction and Preparation
A vast amount of ICA and ICS can be successfully extracted from Epimedium koreanum, another herb often used in traditional Chinese medicine. As a result of purification with a two-phase chloroform-methanol-water solvent system from 200 mg of extract, 46.5 mg of ICA, and 17.7 mg of ICS were obtained. By means of high-speed counter-current chromatography, a single-step separation process resulted in a high-purity (<98%) product [16]. The low content of ICS found in the extraction showed the necessity for another method capable of extracting it with a significantly higher yield. One of the most viable solutions for preparing ICS is the enzymatic hydrolysis of the ICA. With this process, 98% of the icariin was transformed into ICS. This technique consists of a reaction between ICA and β-glucosidase and an extraction with ethyl acetate [17]. In order to prepare a higher amount of ICS, a laboratory pilot scale (500 mL) of enzymatic hydrolysis was successfully developed, which resulted in a 91% yield [18]. Recent studies found an eco-efficient industrial preparation to produce ICS from ICA via enzymatic hydrolysis. The main difference in preparation was achieved by substituting the β-glucosidase enzyme with β-glucanase. The conversion ratio of ICA was 99%, which exceeded the previous ratios [19]. Viable industrial production of icaritin originated from the discovery of a catalytic enzyme, a glycosidase from Aspergillus nidulans, which made possible the biotransformation of epimedin C into ICT [20]. Further studies revealed possibilities for the bioconversion of ICA into ICT, which is a cost-effective method with a high molecular conversion rate (91.2%) [21].
2.2. Medicinal Properties
Thanks to their outstanding medicinal properties in preventing and curing many common health issues, icariin and its derivates have garnered great interest in terms of drug development.
One of the most important medicinal properties of icariin and its derivates is its anti-cancer effects. Although a great decrease in mortality rate has been achieved with the most recent adjuvant chemotherapy regimens, the side effects and toxicity of this procedure are still notable. Hence, there is a high demand for the development of a mechanism-based nontoxic agent that could be applied during patients’ treatment [22]. ICS has shown great potential as a new anti-tumor agent, due to its ability to potentiate paclitaxel-induced apoptosis in melanoma cells. Compared to 5-Fu, a chemosynthetic anticancer drug, icariside II showed stronger anticancer activity on Hela cells, where its IC50 was 10 µM for ICS, while in the case of 5-Fu, IC50 = 31.1 µM [5]. The apoptotic mechanism was also proven on human prostate cancer cells, which demonstrated the great potential for ICS to be used as an effective cancer chemotherapeutic agent [23]. Animal experiments demonstrated that ICS successfully inhibited cervical cancer cell proliferation and promoted apoptosis; therefore, ICS is a promising agent for use in cervical cancer therapies [24]. The anti-leukemic activity of icariin was thoroughly studied and its potential in offering a new treatment option for patients was confirmed [25][26]. As the main metabolite of icariin, icariside II also showed anti-leukemic effects in vitro; consequently, new opportunities for further studies were created [27]. Recently, in vitro experiments proved that ICS is able to promote immune cells to kill the rapidly growing cancer cells [28]. In vivo studies showed a great reduction in the weight of mice liver carcinoma, which was achieved with ICS treatment. The reduction compared to 5-Fu was 50% greater [6]. Icariside II contributed to the anti-proliferative and anti-apoptosis effect of thalidomide. Therefore, in combination with clinically used drugs, it can have an enhanced therapeutic effect in the treatment of multiple myeloma [29].
Recent studies [30][31] highlighted the importance of icariin and its derivates in drug development and in auxiliary therapies to cure different cardiovascular diseases [32]. A 26-week course of therapy, administered to spontaneously hypertensive rats, proved that ICS successfully reduced blood pressure and improved the ventricular wall thickness [33].
Ever since the ancient Chinese era, Epimedium extract has been widely used as an aphrodisiac. In the past few years, many studies have investigated and proved the role of icariin and icariside II in the treatment of erectile dysfunction [34]. An improvement in erectile function was observed in spontaneously hypertensive rats, since icariin successfully decreased the content of endothelial microparticles in their blood, from 1.58% in the control group to 1.01% [35]. In vitro studies indicated that icariin could lead to increased testosterone secretion [36]. Further in vivo experiments proved that rats treated with an appropriate dose of icariin had a significantly (p < 0.001) higher testosterone level. This treatment also showed a slightly (p < 0.01) greater sperm count than in the control group. It was indicated that icariin contributes to improved testosterone and sperm production by gene regulation [37].
The degeneration or death of nerve cells can lead to neurodegenerative diseases (such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and multiple sclerosis [38]), which so far are incurable. Icariside II showed an approximate efficacy to match that of donepezil, which is one of the few drugs approved by the Food and Drug Administration (FDA) to slow the worsening of the symptoms of Alzheimer’s disease [7]. Icariside II can be used in the treatment of excitotoxicity-related diseases, due to its potential as a protective agent for the hippocampal neurons [39]. Several studies have indicated that ICS showed promising results in the prevention [40] and treatment [41] of brain ischemic injury. The currently prescribed non-steroidal anti-inflammatory drugs are highly debated, due to their inconsistent results and toxic effects. Therefore, a new treatment was developed to increase the efficacy of the treatment and reduce its toxicity. ICS is also a possible protective non-toxic agent for acute neuroinflammation-related diseases [42].
Osteoporosis is a health condition that weakens bones by causing low bone mass and the deterioration of bone tissue [43]. Recent studies have shown that icariin and its derivates are potential drugs for postmenopausal osteoporosis treatment. In vivo experiments on ovariectomized rats showed that icariside II can promote the bone marrow stromal cells’ differentiation into osteoblasts and enhance osteoblast activity [44]. Recent studies showed that icariin treatment on fractured bones can accelerate healing [45][46]. In vivo experiments on different ages of rats with induced tibia fractures showed that icariin promoted fracture healing by activating the BMP-2/Smad5/Runx2 pathway [47].
It is necessary to highlight that, reportedly, ICA, ICS, and ICT showed low to no toxicity [4][20][48].
The above-mentioned medicinal properties of icariin and its derivates are summarized in Figure 1.
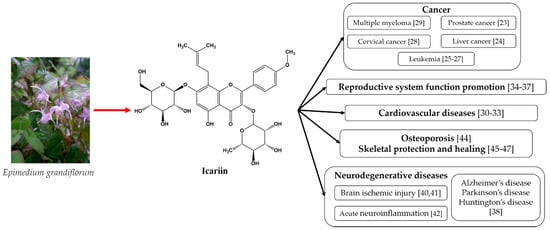
Figure 1. The medicinal properties of icariin [49].
As can be seen from Figure 1, icariin’s most well-known therapeutic properties are its anti-tumor effects, cardiovascular function improvement, sexual dysfunction amelioration, and neuroprotective effects. All of these properties emphasize the need for bioavailability improvements in these materials.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23147519
References
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905.
- Gonzales, G.B.; Van Camp, J.; Smagghe, G.; Raes, K.; Mackie, A. Flavonoid–gastrointestinal mucus interaction and its potential role in regulating flavonoid bioavailability and mucosal biophysical properties. Food Res. Int. 2016, 88, 342–347.
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126.
- He, C.; Wang, Z.; Shi, J. Chapter Seven—Pharmacological effects of icariin. In Advances in Pharmacology; Du, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 179–203.
- Wu, J.; Guan, M.; Wong, P.F.; Yu, H.; Dong, J.; Xu, J. Icariside II potentiates paclitaxel-induced apoptosis in human melanoma A375 cells by inhibiting TLR4 signaling pathway. Food Chem. Toxicol. 2012, 50, 3019–3024.
- Zhang, C.; Shi, Y.-M.; Xia, Y.-Z.; Guo, C.; Yang, L.; Kong, L.-Y. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma. Cancer Lett. 2015, 366, 19–31.
- Adlimoghaddam, A.; Neuendorff, M.; Roy, B.; Albensi, B.C. A review of clinical treatment considerations of donepezil in severe Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 876–888.
- Vazhappilly, C.G.; Amararathna, M.; Cyril, A.C.; Linger, R.; Matar, R.; Merheb, M.; Ramadan, W.S.; Radhakrishnan, R.; Rupasinghe, H.P.V. Current methodologies to refine bioavailability, delivery, and therapeutic efficacy of plant flavonoids in cancer treatment. J. Nutr. Biochem. 2021, 94, 108623.
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An outline for the pharmacological effect of icariin in the nervous system. Eur. J. Pharmacol. 2019, 842, 20–32.
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642.
- Wu, C.; Zhang, J.; Zhou, T.; Guo, B.; Wang, Y.; Hou, J. Simultaneous determination of seven flavonoids in dog plasma by ultra-performance liquid chromatography–tandem mass spectrometry and its application to a bioequivalence study of bioactive components in Herba Epimedii and Er-Xian Decoction. J. Pharm. Biomed. Anal. 2011, 54, 186–191.
- Arief, Z.M.; Shawl, A.S.; Munshi, A.H. Altitudinal variation in pharmacologically active compounds of wild and cultivated populations of Epimedium elatum. J. Appl Res. Med. Aromat Plants 2016, 3, 48–51.
- Wu, C.S.; Guo, B.L.; Sheng, Y.X.; Zhang, J.L. Simultaneous determination of seven flavonoids in Epimedium by liquid chromatography–tandem mass spectrometry method. Chin. Chem. Lett. 2008, 19, 329–332.
- Wu, H.; Kim, M.; Han, J. Icariin metabolism by human intestinal microflora. Molecules 2016, 21, 1158.
- Cheng, T.; Sheng, T.; Yi, Y.; Zhang, T.; Han, H. Metabolism profiles of icariin in rats using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry and in vitro enzymatic study. J. Chromatogr. B 2016, 1033, 353–360.
- Liu, R.; Li, A.; Sun, A.; Cui, J.; Kong, L. Preparative isolation and purification of three flavonoids from the Chinese medicinal plant Epimedium koreamum Nakai by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1064, 53–57.
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442.
- Park, J.-S.; Park, H.-Y.; Rho, H.-S.; Ahn, S.-M.; Kim, D.-H.; Chang, I.-S. Statistically designed enzymatic hydrolysis for optimized production of icariside II as a novel melanogenesis inhibitor. J. Microbiol. Biotechnol. 2008, 18, 110–117.
- Shen, Y.; Wang, M.; Zhou, J.; Chen, Y.; Xu, L.; Wu, M.; Xia, G.; Tam, J.P.; Yu, J.; Teng, X. Eco-efficient biphasic enzymatic hydrolysis for the green production of rare baohuoside I. Enzyme Microb. Technol. 2019, 131, 109431.
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180.
- Li, Q.; Ge, L.; Zheng, D.; Zhang, X.; Zhao, L. Screening and characterization of a GH78 α-l-rhamnosidase from Aspergillus terreus and its application in the bioconversion of icariin to icaritin with recombinant β-glucosidase. Enzyme Microb. Technol. 2022, 153, 109940.
- Yang, L.; Zhang, C.; Kong, L.-y. Blockade of epidermal growth factor receptor/mammalian target of rapamycin pathway by Icariside II results in reduced cell proliferation of osteosarcoma cells. Food Chem. Toxicol. 2014, 73, 7–16.
- Lee, K.-S.; Lee, H.-J.; Ahn, K.S.; Kim, S.-H.; Nam, D.; Kim, D.K.; Choi, D.-Y.; Ahn, K.-S.; Lu, J.; Kim, S.-H. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009, 280, 93–100.
- Sun, Y.-S.; Thakur, K.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. Icariside II inhibits tumorigenesis via inhibiting AKT/Cyclin E/CDK 2 pathway and activating mitochondria-dependent pathway. Pharmacol. Res. 2020, 152, 104616.
- Chen, M.; Wu, J.; Luo, Q.; Mo, S.; Lyu, Y.; Wei, Y.; Dong, J. The anticancer properties of Herba Epimedii and its main bioactive componentsicariin and icariside II. Nutrients 2016, 8, 563.
- Wang, Z.; Zhang, H.; Dai, L.; Song, T.; Li, P.; Liu, Y.; Wang, L. Arsenic trioxide and icariin show synergistic anti-leukemic activity. Cell Biochem. Biophys. 2015, 73, 213–219.
- Yang, J.; Lan, J.; Du, H.; Zhang, X.; Li, A.; Zhang, X.; Liu, Y.; Zhang, J.; Zhang, C.; Ding, Y. Icariside II induces cell cycle arrest and differentiation via TLR8/MyD88/p38 pathway in acute myeloid leukemia cells. Eur. J. Pharmacol. 2019, 846, 12–22.
- Sun, Y.-S.; Thakur, K.; Hu, F.; Cespedes-Acuña, C.L.; Zhang, J.-G.; Wei, Z.-J. Icariside II suppresses cervical cancer cell migration through JNK modulated matrix metalloproteinase-2/9 inhibition in vitro and in vivo. Biomed. Pharmacother. 2020, 125, 110013.
- Kim, S.-H.; Ahn, K.S.; Jeong, S.-J.; Kwon, T.-R.; Jung, J.H.; Yun, S.-M.; Han, I.; Lee, S.-G.; Kim, D.K.; Kang, M. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur. J. Pharmacol. 2011, 654, 10–16.
- Sharma, S.; Khan, V.; Dhyani, N.; Najmi, A.; Haque, S. Icariin attenuates isoproterenol-induced cardiac toxicity in Wistar rats via modulating cGMP level and NF-κB signaling cascade. Hum. Exp. Toxicol. 2020, 39, 117–126.
- Ren, L.; Wang, Z.; Hua, Q.; Xie, H.; Tang, S. Icaritin prevents vascular calcification in mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 35–39.
- Liu, X.-Y.; Liao, H.-h.; Feng, H.; Zhang, N.; Yang, J.-j.; Li, W.-j.; Chen, S.; Deng, W.; Tang, Q.-Z. Icariside II attenuates cardiac remodeling via AMPKα2/mTORC1 in vivo and in vitro. J. Pharmacol. Sci. 2018, 138, 38–45.
- Fu, S.; Li, Y.-L.; Wu, Y.-T.; Yue, Y.; Qian, Z.-Q.; Yang, D.-L. Icariside II attenuates myocardial fibrosis by inhibiting nuclear factor-κB and the TGF-β1/Smad2 signalling pathway in spontaneously hypertensive rats. Biomed. Pharmacother. 2018, 100, 64–71.
- Zheng, T.; Zhang, T.; Zhang, W.; Lv, K.; Jia, D.; Yang, F.; Sun, Y.; Lian, J.; Wang, R. Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed. Pharmacother. 2020, 125, 109888.
- Li, X.; Yang, H.F.; Chen, Y.; Pei, L.J.; Jiang, R. Effect of the icariin on endothelial microparticles, endothelial progenitor cells, platelets, and erectile function in spontaneously hypertensive rats. Andrology 2022, 10, 576–584.
- Xiong, Y.-B.; Zhou, C.-H. The effect of extracts from Herba Epimedii and Semen Cuscutae on the function of male reproduction. Chin. Pharm. J. 1994, 29, 89.
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of icariin on reproductive functions in male rats. Molecules 2014, 19, 9502–9514.
- Zhu, L.; Li, D.; Chen, C.; Wang, G.; Shi, J.; Zhang, F. Activation of Nrf2 signaling by Icariin protects against 6-OHDA-induced neurotoxicity. Biotechnol. Appl. Biochem. 2019, 66, 465–471.
- He, L.; Deng, Y.; Gao, J.; Zeng, L.; Gong, Q. Icariside II ameliorates ibotenic acid-induced cognitive impairment and apoptotic response via modulation of MAPK pathway in rats. Phytomedicine 2018, 41, 74–81.
- Yan, B.-Y.; Pan, C.-S.; Mao, X.-W.; Yang, L.; Liu, Y.-Y.; Yan, L.; Mu, H.-N.; Wang, C.-S.; Sun, K.; Liao, F.-L. Icariside II improves cerebral microcirculatory disturbance and alleviates hippocampal injury in gerbils after ischemia–reperfusion. Brain Res. 2014, 1573, 63–73.
- Deng, Y.; Xiong, D.; Yin, C.; Liu, B.; Shi, J.; Gong, Q. Icariside II protects against cerebral ischemia–reperfusion injury in rats via nuclear factor-κB inhibition and peroxisome proliferator-activated receptor up-regulation. Neurochem. Int. 2016, 96, 56–61.
- Zhou, J.; Deng, Y.; Li, F.; Yin, C.; Shi, J.; Gong, Q. Icariside II attenuates lipopolysaccharide-induced neuroinflammation through inhibiting TLR4/MyD88/NF-κB pathway in rats. Biomed. Pharmacother. 2019, 111, 315–324.
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069.
- Xi, Y.; Jiang, T.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Ye, X. Preliminary studies on the anti-osteoporosis activity of Baohuoside I. Biomed. Pharmacother. 2019, 115, 108850.
- Zhang, Y.; Han, B.; Wei, Y.; Jing, J.; Li, J. Icariin promotes fracture healing in ovariectomized rats. Med. Sci. Monit. 2020, 26, e924554-1–e924554-8.
- Zhang, X.-Y.; Chen, Y.-P.; Zhang, C.; Zhang, X.; Xia, T.; Han, J.; Yang, N.; Song, S.-L.; Xu, C.-H. Icariin accelerates fracture healing via activation of the WNT1/β-catenin osteogenic signaling pathway. Curr. Pharm. Biotechnol. 2020, 21, 1645–1653.
- Zhang, X.; Chen, Y.; Zhang, C.; Zhang, X.; Xia, T.; Han, J.; Song, S.; Xu, C.; Chen, F. Effects of icariin on the fracture healing in young and old rats and its mechanism. Pharm. Biol. 2021, 59, 1243–1253.
- Xu, F.; Wu, Q.; Li, L.; Gong, J.; Huo, R.; Cui, W. Icariside II: Anticancer Potential and Molecular Targets in Solid Cancers. Front. Pharmacol. 2021, 12, 663776.
- Epimedium Grandiflorum. Available online: https://commons.wikimedia.org/wiki/File:Epimedium_grandiflorum.JPG (accessed on 7 June 2022).
This entry is offline, you can click here to edit this entry!
