Deubiquitinating enzymes (DUBs) are a group of proteases that are important for maintaining cell homeostasis by regulating the balance between ubiquitination and deubiquitination. As the only known metalloproteinase family of DUBs, JAB1/MPN/Mov34 metalloenzymes (JAMMs) are specifically associated with tumorigenesis and immunological and inflammatory diseases at multiple levels. The far smaller numbers and distinct catalytic mechanism of JAMMs render them attractive drug targets. Currently, several JAMM inhibitors have been successfully developed and have shown promising therapeutic efficacy.
1. Introduction
Protein ubiquitination, defined as a process that covalently conjugates ubiquitin to the target protein, is one of the most powerful post-translational modifications regulating virtually all cellular processes, such as cell death, cell cycle, and DNA repair [
1,
2,
3,
4]. Ubiquitin is a 76-amino-acid, 8-kDa polypeptide with a conserved sequence that is present universally and ubiquitously in eukaryotes [
5,
6]. Full-length ubiquitin contains eight ubiquitination sites, including seven lysine residues (K6, K11, K27, K29, K33, K48, K63) and an N-terminal methionine residue (M1) (
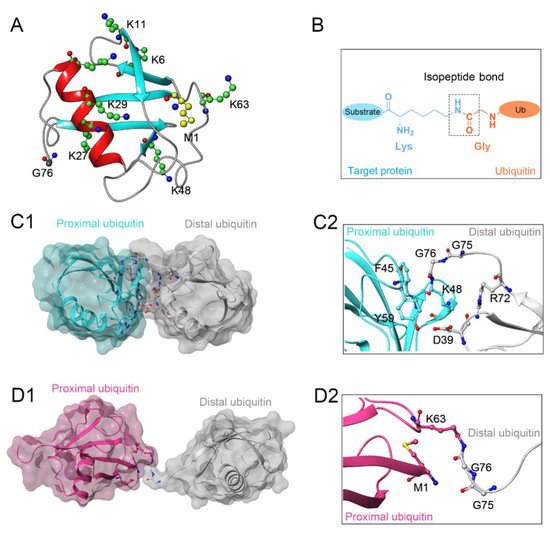
Figure 1A) [
7,
8]. Under the sequential action of ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3), an isopeptide linkage is formed between the carboxyl group of the ubiquitin C-terminal glycine and the ε-amino group of the target protein lysine (
Figure 1B) [
9,
10]. Then, the Gly 76 of additional ubiquitin molecules (called distal ubiquitin) can be covalently attached to the ubiquitination sites in ubiquitin itself (called proximal ubiquitin), to produce polyubiquitin chains [
11]. As a result, various types of protein ubiquitination are formed, which determine the fate of ubiquitinated substrates [
6,
12]. For example, polyubiquitin chains linked via the K48 of internal ubiquitin groups are used for protein degradation signaling by the ubiquitin-proteasome system (
Figure 1C1,C2), whereas K63-linked polyubiquitin chains, presenting different architecture, play proteasome-independent roles in various intracellular events, such as inflammatory signaling, DNA repair, ribosomal protein synthesis, endocytosis, and vesicular trafficking (
Figure 1D1,D2) [
13,
14,
15]. Additionally, some other ubiquitin-like modifications, such as small ubiquitin-like modifier (SUMO), neuronal precursor cell-expressed developmentally downregulated protein 8 (NEDD8), and interferon stimulated gene 15 (ISG15), can also be ligated to target proteins in a process similar to ubiquitylation, mostly to provide nondegradative signals [
16].
Figure 1. (A) Crystal structure of human ubiquitin (PDB ID: 1UBQ). Seven lysine residues and an N-terminal methionine residue are colored green and yellow, respectively. (B) The isopeptide bond between the ubiquitin glycine residue (orange) and the target protein lysine residue (blue). (C1,C2) The overall structure and local conformation of K48-linked polyubiquitin chains (PDB ID: 1TBE). (D1,D2) The overall structure and local conformation of K63-linked polyubiquitin chains (PDB ID: 3HM3). The distal and proximal ubiquitin are colored gray and cyan, respectively.
To date, the human genome encodes nearly 600 E3 ligases while only approximately 100 deubiquitinating enzymes (DUBs) have been identified, clustered in the following 7 families: 56 ubiquitin-specific peptidases (USPs), 17 ovarian tumor proteases (OTUs), 12 JAB1/MPN/Mov34 metalloenzymes (JAMMs), 5 motif interacting with ubiquitin-containing novel DUB family proteases (MINDYs), 4 ubiquitin C-terminal hydroxylases (UCHs), 4 Machado-Josephin domain proteases (MJDs), and 1 zinc finger-containing ubiquitin peptidase 1 (ZUP1) [
17,
18,
19]. Six of these seven families are cysteine proteases, whereas only the JAMM family are zinc-dependent metalloproteinases. In the case of cysteine protease DUBs, the catalytic domains contain a highly conserved catalytic triad comprising cysteine and nearby histidine and aspartate residues [
8,
20]. In contrast, metalloprotease DUBs coordinate Zn
2+ ion with histidine, aspartate, and serine residues to attack the isopeptide bond by activating a water molecule [
21].
Dysfunction of the ubiquitin system, especially DUBs, has been recognized as a contributing factor in the origin of many human diseases, such as cancer, inflammatory diseases, and neurological diseases [
22,
23]. Notably, there has been a recent expansion of drug discovery programs targeting JAMMs. Unlike the large number of cysteine protease DUBs (~90), as few as 12 JAMMs have been identified in the human genome, among which only 7 (AMSH, AMSH-LP, BRCC36, eIF3h, Rpn11, MYSM1, and CSN5) exhibit isopeptidase activity toward ubiquitin chains [
17,
24]. Furthermore, multiple JAMM-related signaling pathways, such as DNA damage control (BRCC36) [
25], endocytosis (AMSH, AMSH-LP) [
26,
27], protein biosynthesis (eIF3h) [
28], and protein degradation (Rpn11, CSN5) [
29], have been confirmed to be associated with numerous diseases, including tumorigenesis and immunological and inflammatory disorders. The much fewer numbers, distinct catalytic mechanism, and, specifically, association with diseases render JAMMs a new class of potential drug targets [
30]. To gain an in-depth understanding of JAMMs, this review emphatically discusses the structural basis, catalytic mechanism, and diverse functions with a focus on JAMM family proteins, including AMSH, AMSH-LP, BRCC36, Rpn11, and CSN5. We also summarize the current reported inhibitors targeting JAMM family members.
2. Structural Characteristic of JAMMs
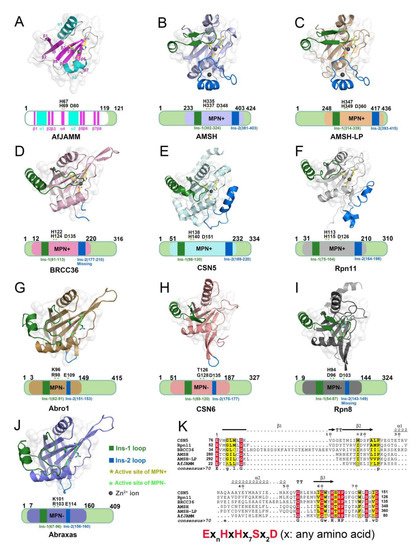
The MPN (Mpr1/Pad1 N-terminal) domain is a striking characteristic of the JAMM family. In 2004, Ambroggio et al. first reported the crystal structure of the MPN domain protein (PDB ID: 1R5X) from a prokaryotic organism
Archaeoglobus fulgidus AF2198 (AfJAMM) [
31]. They revealed that the MPN domain of AfJAMM consisted of an eight-stranded β sheet (β1–β8), flanked by a long α helix (α1) between the first and second strand, and a short α helix (α2) between the fourth and fifth strand (
Figure 2A). Subsequently, an increasing number of crystal structures were resolved and the MPN domain proteins could then be further divided into two subfamilies: (1) the MPN+ family, with isopeptidase activity, characterized by a zinc-coordinating JAMM motif (Ex
nHxHx
7Sx
2D) (where x represents any amino acid residue) (
Figure 2K); and (2) the MPN– family, without catalytic activity, serving as scaffolds in some JAMM multi-subunit complexes [
32,
33,
34].
Figure 2. Structural characteristics of JAMM MPN domain mentioned in this review. (A) Crystal structure of AfJAMM (PDB ID: 1R5X). AfJAMM has a typical MPN domain containing an eight-stranded β sheet (β1–β8) (fuchsia), a long α helix (α1), and a short α helix (α2) (cyan). (B–J) Crystal structure of AMSH (PDB ID: 3RZU) (light blue), AMSH-LP (PDB ID: 2ZNV) (wheat), BRCC36 (PDB ID: 6H3C) (light pink), CSN5 (PDB ID: 4F7O) (pale cyan), Rpn11 (PDB ID: 4O8X) (gray), Abro1 (PDB ID: 6H3C) (sand), CSN6 (PDB ID: 4D10) (salmon), Rpn8 (PDB ID: 4O8X) (light black), and Abraxas (PDB ID: 6GVW) (slate) MPN domain. All the Ins-1 and Ins-2 loop are colored deep green and blue, respectively. The yellow and green asterisks represent active sites of MPN+ and MPN–, respectively. The black round represents Zn2+ ion. (K) The zinc-coordinating JAMM motif of MPN+ (ExnHxHx7Sx2D) (where x is any amino acid residue).
Most JAMMs possess two unique insertions, referred to as Ins-1 and Ins-2, which are considered to play important roles in the recognition and binding of ubiquitinated protein substrates [
24]. The Ins-1 segment forms one ridge of the substrate-binding groove to assist in the proper positioning of the C-terminal ubiquitin tail for catalysis while the Ins-2 region contributes to the productive substrate positioning [
35].
3. Catalytic Mechanism of JAMMs
So far, 7 of the 12 JAMMs (AMSH, AMSH-LP, BRCC36, eIF3h, Rpn11, CSN5, and MYSM1) in the human genome belong to the MPN+ subfamily and have DUB activity toward proteins while the remaining 5 JAMMs (Abraxas, Abro1, CSN6, eIF3f, and Rpn8) belong to the MPN− subfamily [
44,
45]. Interestingly, most of these JAMMs require the formation of multi-subunit complexes to exert their isopeptidase activities, including Rpn11 and Rpn8 of the 26S proteasome [
29], CSN5 and CSN6 of the COP9 signalosome [
46], eIF3f and eIF3h of the human translation initiation factor 3 (eIF3) [
47], BRCC36 and Abraxas of the BRCA1-A complex [
48], and BRCC36 and Abro1 of the BRISC complex [
49]. There are, of course, exceptions, such as AMSH and AMSH-LP, which can cleave K63-linked ubiquitin chains independent of protein partners [
50]. Sato et al. resolved the crystal structure of AMSH-LP
E292A-ubiquitin complex (PDB ID: 2ZNV) from
H. sapiens and proposed the catalytic mechanism of JAMMs, which was probably similar to that of thermolysin (
Figure 3H) [
26,
51].
First, the zinc-bound catalytic water is deprotonated by an active site Glu 292 and subsequently performs a nucleophilic attack on the substrate peptide carbonyl. Then, the negative charge on the peptide carbonyl oxygen is stabilized by the Zn
2+ ion and His 347, His 349, Ser 357, and Asp 360 while the positive charge on the amide nitrogen is stabilized by Glu 292. The reaction then proceeds through an oxyanion tetrahedral intermediate and a second transition state, which results in the cleavage of the peptide N-C bond. With the proton transferring from the amide nitrogen to water, the cleavage of the peptide bond is ultimately completed (
Figure 3I) [
26]. Although the whole amino acid sequences of these seven MPN+ members are highly divergent, the catalytic core region is completely conserved, suggesting that they might employ identical catalytic mechanisms [
30].
4. Structural and Functional Basis of JAMMs
4.1. Functional Basis of AMSH in Receptor Endocytosis
It has recently been shown that AMSH plays a significant role in regulating the endosomal sorting of many cell-surface receptors, which is a highly regulated process for maintaining cellular homeostasis and generating adaptive responses to external stimuli [
52,
53]. Typically, the endocytic trafficking process involves the internalization, endosomal sorting, and lysosomal degradation of cell-surface receptors and is strictly executed by the endosomal sorting complexes required for transport (ESCRT), consisting of at least five macromolecular assemblies termed ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III and vacuolar sorting protein 4 (Vps4) [
54,
55,
56]. It is during this process that AMSH can interact with the components ESCRT-0 and ESCRT-III and so affect the fate of receptors [
16].
Several studies have documented the crucial role of AMSH-mediated deubiquitination in the trafficking of endocytosed receptors, such as receptor-tyrosine kinase epidermal growth factor receptor (EGFR), G protein-coupled receptors (GPCRs), connexins 43 (connexin Cx43), and the inflammasome component NACHT, LRR, and PYD domain-containing protein (NALP7) (
Table 1) [
57,
58,
59,
60,
61,
62]. For example, the E3-ligase c-Cbl has been shown to promote lysosomal degradation of the K63 ubiquitylated EGFR [
63] while AMSH opposes this action and promotes EGFR recycling, thus regulating the balance of the intracellular EGFR content [
59]. In another study, Ribeiro-Rodrigues et al. demonstrated that AMSH could protect gap junctions from degradation by mediating the deubiquitination of Cx43 to regulate intercellular communication [
60]. By linking the DUBs to immune regulation, Mallampalli et al. found that AMSH cleaved K63-linked ubiquitin from NALP7 to increase its intracellular content, leading to inflammasome-dependent IL-1β cleavage and release [
62]. For some important GPCRs, including chemokine receptor CXCR4, protease-activated receptor 2 (PAR
2), and δ-opioid receptor (DOR), AMSH has been reported to regulate their stability and trafficking, as the loss of AMSH catalytic activity can significantly alter the steady-state level of GPCRs [
57,
58,
61]. Overall, AMSH-mediated receptor endocytosis is accomplished through the recognition of specific ubiquitination patterns, specifically multi-monoubiquitination and K63-linked polyubiquitination.
This entry is adapted from the peer-reviewed paper 10.3390/biom12070910