Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sphingosine-1-phosphate (S1P) and S1P receptors (S1PR) are bioactive lipid molecules that are ubiquitously expressed in the human body and play an important role in the immune system.
- S1P
- S1PR1
1. Introduction
Sphingosine-1-phosphate (S1P) and S1P receptors (S1PR) are bioactive lipid molecules that are ubiquitously expressed in the human body and play a fundamental role in the trafficking and activation in the immune system. The first description by Spiegel et al. defined S1P as a signaling molecule that stimulates fibroblast proliferation [1][2]. S1P itself is a physiologic signaling molecule that acts as a ligand for a group of cell surface receptors. There are five different high-affinity cell surface receptors of S1P, which belong to the superfamily of G protein-coupled receptors (GPCR) [3]. Interaction with specific intracellular targets facilitates the propagation of S1P signals and subsequently, upon release, ligation of the five known heptameric GPCR can result both in autocrine and paracrine signaling [4].
The important role played by S1P/S1PR in various cellular functions such as vascular tone, heart rate, preservation of endothelial barrier and fundamentally, in immune cell trafficking have been proven in multiple studies [5]. This wide range of biological functions leads S1P/S1PR to be involved in pathogenesis of immunological and non-immunological conditions. Thus, S1P and S1PR were proposed as therapeutic targets for prevention or treatment of some diseases [6].
2. Mechanism of Action of S1P/S1PR
The cell membrane is composed of a large number of proteins, among them sphingosines, which in turn are the source of many bioactive lipids, including ceramide, sphingosine, S1P and ceramide-1-phosphate [7][8]. S1P is the active terminal derivative of sphingosine metabolism, generated by the action of sphingosine kinase (SKs) [9][10]. SKs are activated by cytokines, immunoglobulins, hormones, and trophic factors [10]. S1P/S1PR complex has a broad range of functions due to the autocrine and paracrine effects and its presence in the blood and the lymphatic system (Figure 1).
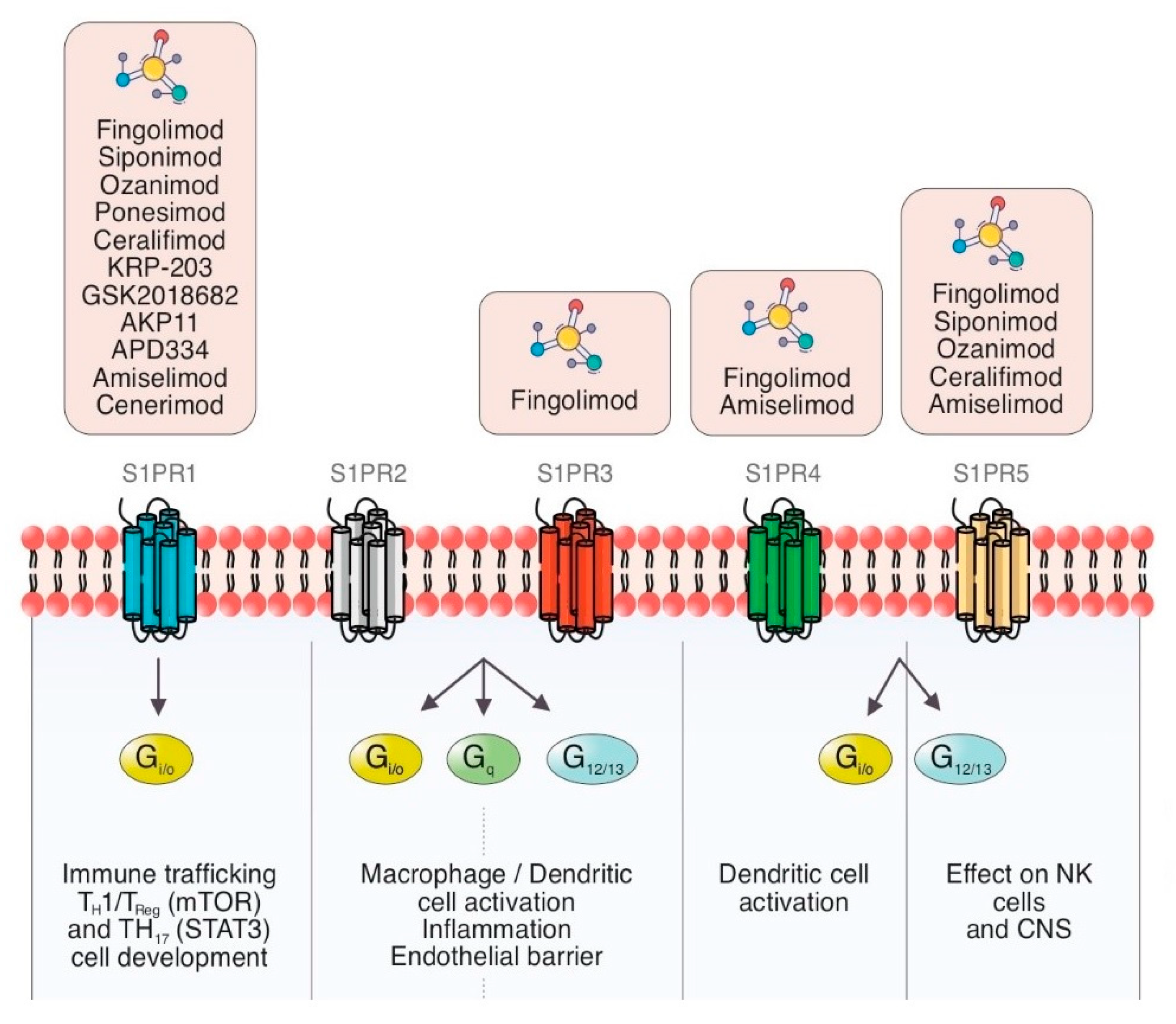
Figure 1. Schematic summary of the current view of S1PR modulators and S1P signaling pathways with cellular therapeutic targets in inflammation and immune processes through different G proteins.
S1P exerts its action through multiple enzymatic reactions in different cell locations, such as endoplasmic reticulum, mitochondria and nucleus [11][12].
The main S1P effect is regulating the lymphocyte egress from secondary lymphatic organs (SLO) into the systemic circulation, being the SLO the main location where its cardinal function is implemented [13][14][15]. To reach an adequate gradient of lymphocytes between blood and the lymph nodes a perfect equilibrium between the synthesis and degradation of S1P is necessary, via sphingosine kinases and sphingosine lyases, respectively. This equilibrium is altered when a modulator such as fingolimod is introduced [16][17].
S1P was initially thought not to have an active function, but this misconception changed when the S1PR was discovered and its characteristics as first messenger were better understood [18][19]. It has been proven that S1P is involved in multiple biological effects due to both its ubiquitous distribution and its pleiotropic effect, not only in the immune system, but also in limb development, neurogenesis, cardiogenesis, and generation and proliferation of vessels [20][21].
As was previously mentioned, S1P functions are mediated by five GPCR expressed in many organs (S1PR1 to S1PR5) [22]. S1PR1 expressed on lymphocytes T and B is implicated in modulating the expression of some pro-inflammatory cytokines [23][24][25]. S1P is involved in mechanisms of immune tolerance and prevention of autoimmunity. There are some ongoing trials about its implication on autoimmune diseases based on these effects [25][26].
S1P is released by cerebral sphingosines in the central nervous system (CNS), and its receptor is expressed by all types of brain cells, including neurons, astrocytes, and oligodendrocytes [27][28]. The discovery of the presence of the S1P/S1PR complex in the CNS was the main factor to figure out that the modulation of the signaling of this complex might have therapeutic implications for neurological disorders, including multiple sclerosis [29][30].
Given all the previously exposed, the effects of S1P can be theoretically divided into both peripheral effect due to the actions exerted on the immune system, and central effect because of its interactions in the central nervous system [31].
3. S1PR Isoforms
3.1. Sphingosine-1-Phosphate Receptor: Isoform 1 (S1PR1)
S1PR1 is ubiquitous and is virtually expressed in every cell line [32]. Its wide distribution is the reason it has multiple biological effects in various organs [32][33]. As outlined above, S1PR-1 has a peripheral effect in the immune system and other organs and a central effect in the CNS [32]. Multiple functions such as actin remodeling, chemotaxis, lymphocyte egress, vascular integrity, organogenesis including angiogenesis, cell growth and proliferation and antimicrobial cytotoxicity are carried out by immune and vascular cells at peripheral level [33][34] (Figure 2). In CNS, S1PR1 is expressed by astrocytes, microglia, oligodendrocytes and neurons [33]. Its effects vary depending on the cellular lineage, not being well understood yet. Nevertheless, it is thought that S1PR1 induces overexpression of glial fibrillary acidic protein (GFAP) levels and morphological changes in neurons, therefore being directly involved in astrocyte proliferation and activation [35]. In neurons, S1PR1 also stimulates the migration of neuronal progenitor cells towards lesion sites [36]. The S1PR1 effects on oligodendrocyte progenitor cells (OPCs) are not properly stablished, but modulators such as fingolimod or siponimod have failed to show remyelination [34].
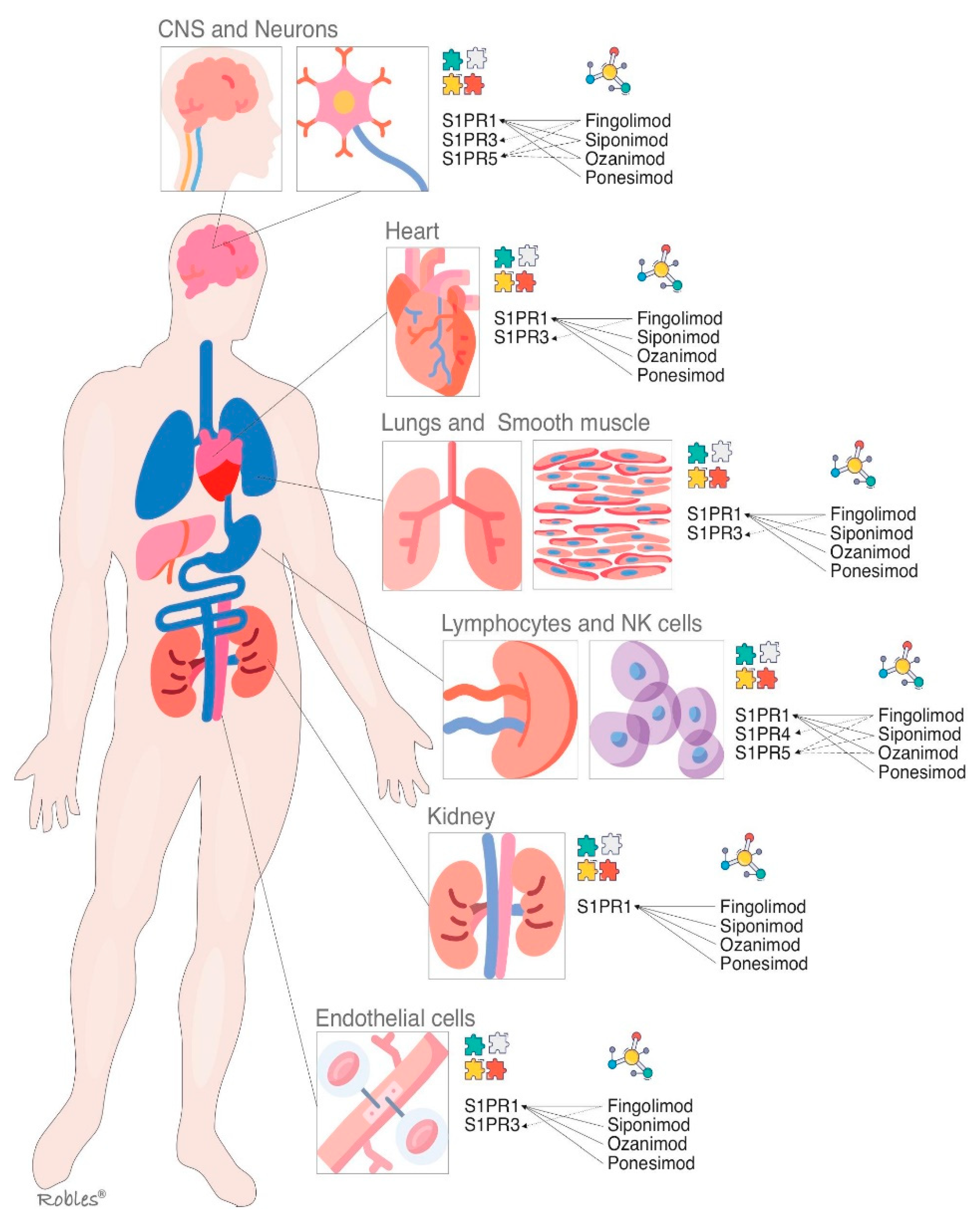
Figure 2. S1P receptors and their main localizations altogether with the S1P modulators and their targets. S1PR = Sphingosine1-phosphate receptor, CNS = central nervous system.
The fingolimod causes down-regulation in multiple lines of cells, so immunosuppressive effect is due to the downregulation of S1PR1 on T cells whereas its action on endothelial cells is responsible for increasing permeability and vascular leakage with the subsequent side effects observed after long-term treatment [36].
S1P1 seems to be a promising pharmacological target that remained to be explored in the different diseases in which it is pathophysiological involved.
3.2. Sphingosine-1-Phosphate Receptor: Isoform 2 (S1PR2)
The S1PR2 binds to S1P with high affinity [37]. S1PR2, as well as other S1PR, signals through various G proteins and is widely expressed in different organs [38]. It is supposed that S1PR2 plays an important role in inhibition of apoptosis, cellular proliferation, actin remodeling and B cells positioning in follicles and in the the development of the heart and the auditory and vestibular system [39][40]. The establishing of endothelial barriers, especially in inner ear and retina are other of its key functions [39]. Nonetheless, these biological effects are poorly understood, thus the development of selective modulators has not been studied sufficiently [40][41].
3.3. Sphingosine-1-Phosphate Receptor: Isoform 3 (S1PR3)
The functions of S1PR3 are still not completely clarified, but an essential role in the regulation of vascular tone by mean of vasodilation has been proposed. It is also involved in cytokine production, in the protection of myocardial ischemia, and in coagulation during inflammatory processes [42] (Figure 2). Moreover, S1PR3 enhances the Notch signaling pathway and is implicated in retinal astrogliosis. The S1PR3 effect on immune system remains controversial as both pro-inflammatory and anti-inflammatory effects have been shown [42][43]. Most of the research performed on S1PR3 is related to vascular contraction, stroke, sepsis, cardiac conductivity, asthma and cancer growth and metastasis formation [44][45].
The fingolimod effect on S1PR3 in the heart conduction system causes a negative chronotropic response [46].
3.4. Sphingosine-1-Phosphate Receptor: Isoform 4 (S1PR4)
The S1PR4 is specifically expressed in SLO, hematopoietic tissue and lungs, where it plays an essential role in lymphocyte signaling, megakaryocyte differentiation and platelet formation and activation [47][48] (Figure 2). In CNS, S1PR4 mediates the activation and maturation of dendritic cells [47]. S1PR4 is a negative regulator of cells proliferation and participates in reducing the secretion of effector cytokines [48]. The functioning of S1PR4 system is currently under investigation, and some novel selective agonists are being studied to better understand its biological importance [49].
3.5. Sphingosine-1-Phosphate Receptor: Isoform 5 (S1PR5)
Oligodendrocytes and myelinating cells of the brain and spleen are the cellular lines, which most widely express this S1PR isoform [50]. S1PR5 is one of the main regulators of natural killer cells egress from both bone marrow and spleen into the blood [51]. Activation of the S1PR5 on oligodendrocytes may have a beneficial effect in MS by protecting the oligodendrocytes from demyelination and cell death [52] (Figure 2). Immune quiescence and blood–brain-barrier integrity are other functions that could be mediated by the expression of S1PR5 in the brain endothelial cells [50][51].
This entry is adapted from the peer-reviewed paper 10.3390/cells11132058
References
- Zhang, H.; Desai, N.N.; Olivera, A.; Seki, T.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991, 114, 155–167.
- Olivera, A.; Spiegel, S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 1993, 365, 557–560.
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594.
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1- phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415.
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155.
- Arish, M.; Alaidarous, M.; Ali, R.; Akhter, Y.; Rub, A. Implication of sphingosine-1-phosphate signaling in diseases: Molecular mechanism and therapeutic strategies. J. Recept. Signal Transduct. Res. 2017, 37, 437–446.
- Leong, W.I.; Saba, J.D. S1P metabolism in cancer and other pathological conditions. Biochimie 2010, 92, 716–723.
- Saba, J.D.; Hla, T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 2004, 94, 724–734.
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 2007, 19, 229–237.
- Snider, A.J.; Orr Gandy, K.A.; Obeid, L.M. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010, 92, 707–715.
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2019, 154, 104170.
- Huwiler, A.; Pfeilschifter, J. New players on the center stage: Sphingosine 1-phosphate and its receptors as drug targets. Biochem. Pharm. 2008, 75, 1893–1900.
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60.
- Kleuser, B.; Maceyka, M.; Milstien, S.; Spiegel, S. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett. 2001, 503, 85–90.
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349.
- Huwiler, A.; Zangemeister-Wittke, U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol. Ther. 2018, 185, 34–49.
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457.
- Bigaud, M.; Guerini, D.; Billich, A.; Bassilana, F.; Brinkmann, V. Second generation S1P pathway modulators: Research strategies and clinical developments. Biochim. Biophys. Acta 2014, 1841, 745–758.
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739.
- Argraves, K.M.; Argraves, W.S. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid. Res. 2007, 48, 2325–2333.
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618.
- Blaho, V.A.; Galvani, S.; Engelbrecht, E.; Liu, C.; Swendeman, S.L.; Kono, M.; Proia, R.L.; Steinman, L.; Han, M.H.; Hla, T. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 2015, 523, 342–346.
- Chiba, K.; Hoshino, Y.; Suzuki, C.; Masubuchi, Y.; Yanagawa, Y.; Ohtsuki, M.; Sasaki, S.; Fujita, T. FTY720, a novel immunosuppressant possessing unique mechanisms. I. Prolongation of skin allograft survival and synergistic effect in combination with cyclosporine in rats. Transpl. Proc. 1996, 28, 1056–1059.
- Kihara, A.; Anada, Y.; Igarashi, Y. Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J. Biol. Chem. 2006, 281, 4532–4539.
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500.
- Spiegel, S.; Milstien, S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta 2000, 1484, 107–116.
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360.
- Oo, M.L.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089.
- Mullershausen, F.; Zecri, F.; Cetin, C.; Billich, A.; Guerini, D.; Seuwen, K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 2009, 5, 428–434.
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105.
- Foss, F.W., Jr.; Mathews, T.P.; Kharel, Y.; Kennedy, P.C.; Snyder, A.H.; Davis, M.D.; Lynch, K.R.; Macdonald, T.L. Synthesis and biological evaluation of sphingosine kinase substrates as sphingosine-1-phosphate receptor prodrugs. Bioorg. Med. Chem. 2009, 17, 6123–6136.
- Gaire, B.P.; Lee, C.H.; Sapkota, A.; Lee, S.Y.; Chun, J.; Cho, H.J.; Nam, T.G.; Choi, J.W. Identification of Sphingosine 1-Phosphate Receptor Subtype 1 (S1P1) as a Pathogenic Factor in Transient Focal Cerebral Ischemia. Mol. Neurobiol. 2018, 55, 2320–2332.
- Pan, S.; Mi, Y.; Pally, C.; Beerli, C.; Chen, A.; Guerini, D.; Hinterding, K.; Nuesslein-Hildesheim, B.; Tuntland, T.; Lefebvre, S.; et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 2006, 13, 1227–1234.
- Quancard, J.; Bollbuck, B.; Janser, P.; Angst, D.; Berst, F.; Buehlmayer, P.; Streiff, M.; Beerli, C.; Brinkmann, V.; Guerini, D.; et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem. Biol. 2012, 19, 1142–1451.
- Blankenbach, K.V.; Schwalm, S.; Pfeilschifter, J.; Meyer, Z.; Heringdorf, D. Sphingosine-1-Phosphate Receptor-2 Antagonists: Therapeutic Potential and Potential Risks. Front. Pharmacol. 2016, 7, 167.
- Bigaud, M.; Dincer, Z.; Bollbuck, B.; Dawson, J.; Beckmann, N.; Beerli, C.; Fishli-Cavelti, G.; Nahler, M.; Angst, D.; Janser, P.; et al. Pathophysiological Consequences of a Break in S1P1-Dependent Homeostasis of Vascular Permeability Revealed by S1P1 Competitive Antagonism. PLoS ONE 2016, 11, e0168252.
- Okazaki, H.; Ishizaka, N.; Sakurai, T.; Kurokawa, K.; Goto, K.; Kumada, M.; Takuwa, Y. Molecular Cloning of a Novel Putative G Protein-Coupled Receptor Expressed in the Cardiovascular System. Biochem. Biophys. Res. Commun. 1993, 190, 1104–1109.
- Skoura, A.; Sanchez, T.; Claffey, K.; Mandala, S.M.; Proia, R.L.; Hla, T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Investig. 2007, 117, 2506–2516.
- Kono, M.; Belyantseva, I.A.; Skoura, A.; Frolenkov, G.I.; Starost, M.F.; Dreier, J.L.; Lidington, D.; Bolz, S.S.; Friedman, T.B.; Hla, T.; et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 2007, 282, 10690–10696.
- Burczyk, M.; Burkhalter, M.D.; Blätte, T.; Matysik, S.; Caron, M.G.; Barak, L.S.; Philipp, M. Phenotypic regulation of the sphingosine 1-phosphate receptor miles apart by G protein-coupled receptor kinase 2. Biochemistry 2015, 54, 765–775.
- Skoura, A.; Michaud, J.; Im, D.S.; Thangada, S.; Xiong, Y.; Smith, J.D.; Hla, T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Thromb. Vasc. Biol. 2011, 31, 81–85.
- Murakami, A.; Takasugi, H.; Ohnuma, S.; Koide, Y.; Sakurai, A.; Takeda, S.; Hasegawa, T.; Sasamori, J.; Konno, T.; Hayashi, K.; et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol. Pharmacol. 2010, 77, 704–713.
- Awojoodu, A.O.; Ogle, M.E.; Sefcik, L.S.; Bowers, D.T.; Martin, K.; Brayman, K.L.; Lynch, K.R.; Peirce-Cottler, S.M.; Botchwey, E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 13785–13790.
- Murakami, K.; Kohno, M.; Kadoya, M.; Nagahara, H.; Fujii, W.; Seno, T.; Yamamoto, A.; Oda, R.; Fujiwara, H.; Kubo, T.; et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS ONE 2014, 9, e106792.
- Niessen, F.; Schaffner, F.; Furlan-Freguia, C.; Pawlinski, R.; Bhattacharjee, G.; Chun, J.; Derian, C.K.; Andrade-Gordon, P.; Rosen, H.; Ruf, W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 2008, 452, 654–658.
- Sanna, M.G.; Vincent, K.P.; Repetto, E.; Nguyen, N.; Brown, S.J.; Abgaryan, L.; Riley, S.W.; Leaf, N.B.; Cahalan, S.M.; Kiosses, W.B.; et al. Bitopic Sphingosine 1-Phosphate Receptor 3 (S1P3) Antagonist Rescue from Complete Heart Block: Pharmacological and Genetic Evidence for Direct S1P3 Regulation of Mouse Cardiac Conduction. Mol. Pharmacol. 2016, 89, 176–186.
- Golfier, S.; Kondo, S.; Schulze, T.; Takeuchi, T.; Vassileva, G.; Achtman, A.H.; Gräler, M.H.; Abbondanzo, S.J.; Wiekowski, M.; Kremmer, E.; et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010, 24, 4701–4710.
- Gräler, M.H.; Grosse, R.; Kusch, A.; Kremmer, E.; Gudermann, T.; Lipp, M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 2003, 89, 507–519.
- Guerrero, M.; Urbano, M.; Velaparthi, S.; Schaeffer, M.T.; Brown, S.J.; Crisp, M.; Ferguson, J.; Hodder, P.; Rosen, H.; Oldstone, M.; et al. Identification of a novel agonist of the sphingosine 1-phos-phate receptor 4 (S1P4). In Probe Reports from the NIH Molecular Libraries Program; Bethesda: Rockville, MD, USA, 2010.
- Niedernberg, A.; Scherer, C.R.; Busch, A.E.; Kostenis, E. Comparative analysis of human and rat S1P(5) (edg8): Differential expression profiles and sensitivities to antagonists. Biochem. Pharmacol. 2002, 64, 1243–1250.
- Im, D.S.; Clemens, J.; Macdonald, T.L.; Lynch, K.R. Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (Edg-8): Structure-activity relationship of sphingosine1-phosphate receptors. Biochemistry 2001, 40, 14053–14060.
- Jaillard, C.; Harrison, S.; Stankoff, B.; Aigrot, M.S.; Calver, A.R.; Duddy, G.; Walsh, F.S.; Pangalos, M.N.; Arimura, N.; Kaibuchi, K.; et al. Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 2005, 25, 1459–1469.
This entry is offline, you can click here to edit this entry!
