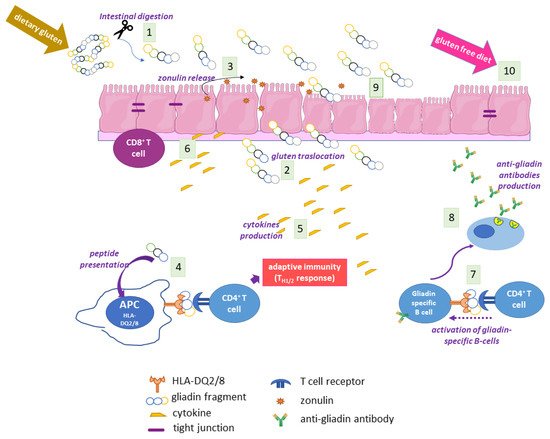
Coeliac disease (CD) is a multifactiorial enteropathy that affects the small intestine of genetically predisposed individuals. A condition of partial to total atrophy, together with crypt hyperplasia and consistent lymphocytic infiltration, characterises the intestinal mucosa of affected patients. The main environmental trigger is a heterogenic proteic component of some dietary cereals, commonly known as gluten. A strong immune response against gluten, both cellular and humoral, is mounted in CD, accompanied by a humoral autoimmune response against self-proteins, in particular type 2 transglutaminase (TG2).
- coeliac disease
- gluten
- autoimmunity
1. Epidemiology and Clinical Manifestations of CD
2. The Main Environmental Trigger in CD: Gluten
3. The Genetics of CD
4. The Adaptive and Innate Immune Response in CD
5. Autoimmunity in CD
6. The Coeliac Cellular Phenotype
7. CD Diagnosis
Enzymatic and Immunofluorescence Assays
8. CD Therapy
9.TG2
The TG family includes transferase enzymes (EC 2.3.2.13), which catalyse the formation of an isopeptide bond involving the g-carboxamide group of a Gln residue (the acyl-donor) and the e-amino group of a Lys residue (the nucleophilic acyl-acceptor), generating an intra- or an inter-protein cross-link [[1]]. The biochemical mechanism of the reaction involves a ping-pong kinetics: first, the g-carboxamide group forms a thiol ester with the Cys residue of the enzyme active site with the release of ammonia, then the acyl intermediate is transferred to the nucleophilic aminic substrate. As alternatives to Lys residues as acyl-acceptors in the crosslinking reaction, TGs can use several low molecular-mass primary amines (spermine, spermidine, putrescine, etc.). In the absence of suitable amines and in the presence of a slightly acidic pH, water can act as nucleophile; thus, TGs are able to deamidate Gln to Glu residues [[1]]. In mammals, nine structurally and functionally related genes encoding TGs have been identified [39]. Among them, only one gene encodes for a protein (band 4.2) without catalytic activity. All other eight mammalian TGs are strictly Ca2+-dependent enzymes and share a high sequence identity around the Cys proteinase-active site, comprising the catalytic triad Cys, His, and Asp. Moreover, the structural organization in four sequential domains is highly conserved in TG isoforms: the N-terminal b-sandwich domain is followed by the catalytic core domain (containing the Ca2+ binding sites) and two C-terminal b-barrel domains [2]].
10. TG2 in CD Pathogenesis
Given the wide range of TG2 enzymatic and non-enzymatic functions and its ubiquitous distribution in tissues and cellular compartments, it is not surprising that TG2 has a role in the development of several human pathological conditions, such as cancer, neurodegenerative disorders, inflammatory and cardiovascular diseases, and autoimmune conditions [54–56]. Regarding CD, TG2 represents the trade union between the main environmental trigger (gluten) and the main genetic component (HLA genes) of CD. This enzyme, whose expression is greatly increased in inflamed intestinal sites both intracellularly (in enterocytes) and extracellularly (in the lamina propria) [[3]], recognizes gluten fragments as preferred substrates and catalyses two crucial reactions: deamidation and transamidation of specific Gln residues.
Gluten Modification: Deamidation
Given the high content of Gln residues in gliadin and other gluten proteins, it is evident that TG2 can use them as Gln-donor substrates in transamidating and deamidating reactions. Deamidation is not a common reaction because it requires specific conditions: a slightly acidic pH and a very low concentration of available amines. When and where these conditions may be realised in cells and tissues are still debated. In a recent work, it has been suggested that TG2 released in gut lumen as a consequence of enterocytes shedding could meet luminal gliadin, and in this way could have direct access to adaptive immune B cells at the level of Peyer’s patches [[4]]. TG2 on the cell surfaces of APCs could deamidate gliadin during its turnover by endocytosis at the level of acidic endocytic vesicles. The recent demonstration that TG2 is more abundant on the cell surface and in early endocytic vesicles of CD skin-derived fibroblasts than of control cells supports this hypothesis [27]. In any case, there is no doubt that TG2 recognises specific Gln residues in gliadin sequences and deamidates them, introducing a net negative charge that greatly increases the affinity with which DQ2/DQ8 heterodimers bind gliadin peptides for presentation to T cells [59]. Indeed, the production of high-resolution X-ray crystal structures of representative deamidated gluten peptides in complex with DQ2 and DQ8 has definitively confirmed the importance of the introduction of a negative charge at a specific location in a gluten peptide interacting with the HLA binding groove [60]. As a consequence, a stronger immune response is evoked. An updated list of CD-relevant deamidated gluten epitopes recognised by CD4+ T cells has recently been reported [[5]]. The majority of listed epitopes are DQ2.5-restricted and mainly belong to the a and g gliadins, but some epitopes also belong to the w-gliadins, glutenins, hordeins, secalins, and avenins. Only a few epitopes are DQ2.2-or DQ8-restricted and also belong to the a and g gliadins and glutenins. This discrepancy is likely due to the fact that most gluten epitopes have been discovered in CD patients positive for HLA-DQ2.5 [[6]]. Thus, it is reasonable to expect that further additional epitopes will be defined in the future.
Gluten Modification: Mechanism of Anti-TG2 Antibody Production
As above reported, TG2 is the main autoantigen in CD. To date, no TG2-specific CD4+ helper T cells have been identified. For this reason, it is supposed that gliadin-specific CD4+ helper T cells are essential in the production of anti-TG2 antibodies at the intestinal level, as a consequence of a cross-linking reaction catalysed by TG2 and involving some gliadin peptides [2]. In this case, reactive Gln residues of gliadin peptides are acyl-donor substrates in the transamidating reaction. The aminic second substrates could be represented by Lys residues of TG2 itself or of other intracellular or surface-membrane proteins. Several models supported by experimental data have tried to explain how this activity could be responsible for the autoimmune response. According to the classic hapten-carrier-like model, TG2 forms isopeptide bonds between itself (involving an autoreactive Lys residue) and gliadin peptides, generating complexes that are internalised, processed, and exposed by HLA-DQ2/8 molecules of TG2-specific B cells [[7]]. Then, these cells activate gluten-specific CD4+ T cells, which in turn stimulate B cells to produce anti-TG2 antibodies. In this regard, several isopeptides between TG2 and gliadin fragments have been identified [[8]]. Recently, du Pré et al. developed an elegant transgenic murine model demonstrating that autoreactive anti-TG2 B cells are able to produce autoantibodies when they receive help from T cells [63]. It has also been proposed that complexes between TG2 and gliadin could also be formed involving the Cys residue of the TG2 active site, with the formation of a thioester bond [[9]]. In any case, the hapten-carrier-like model better explains the occurrence of other autoantibodies besides anti-TG2. Indeed, other autoantigens have been identified (actin, desmin, calreticulin, collagen, etc.) and all can be recognised by TG2 as substrates for forming covalent complexes that could be processed by substrate-specific B-cells [65]. Moreover, this model is compatible with the observation that the autoimmune response gradually decreases when a gluten-free diet is adopted. A revised version of the model proposes that TG2 is able to cross-link gliadin peptides directly to B cell receptors on TG2-specific B lymphocytes [[10]]. In a recent work [[11]], it has been demonstrated that the majority of TG2-specific B cells displayed epitope specificity for TG2 N-terminal sequences, and that cell-surface antibodies with such a specificity allowed TG2-cross-linking activity, leading to the formation of covalent complexes with gliadin and consequential deamidation during antigen presentation. Another model on the generation of TG2 antibodies proposes that neoepitopes formed by the transamidating activity of TG2 targeting gluten peptides and itself could recruit T lymphocytes that escape thymic negative selection [[12]]. Finally, some evidence supports the idea that the autoimmune response against TG2 could involve a mechanism of molecular mimicry regarding viral or bacterial antigens [[13]].
11. Conclusion
It is doubtless that TG2 represents a key enzyme in the pathogenic mechanisms leading to CD development. At the site of intestinal inflammation, where TG2 is typically overexpressed, its canonical enzymatic activity leads to the formation of isopeptide bonds between itself (or other self-proteins) and gluten peptides, thus provoking a strong autoimmune response. Still uncertain is the role of circulating and intestinal anti-TG2 antibodies in contributing to CD onset; however, antibody detection represents an irreplaceable tool for CD screening programs and diagnosis. In particular circumstances (low pH and virtual absence of primary amines) in the CD mucosa, TG2 can modify specific Gln residues of gluten peptides, transforming a neutral residue into an acidic residue (Glu), which is important in the presentation to the immune system by DQ2/8 molecules and the consequent loss of oral tolerance towards gluten. For these evident reasons, TG2 has become the targetof recent pharmaceutical approaches aimed at reducing deamidation. New generations of increasingly specific and potent TG2 inhibitors are under investigation, some of which have reached the point of clinical experimentation. Finally, given the increasing use of food-grade TGs, particularly TGm, as biotechnological tools in a number of industrial applications, some researchers are exploring the possibility of modifying gluten peptides by transamidating them with nucleophilic amines in an attempt to reduce the immunogenicity of the majority of DQ2/8-restricted epitopes. Finally, it is appropriate to underline that TG2 is a multifunctional enzyme with several catalytic activities and non-catalytic functions. Thus, it cannot be excluded that TG2 may have other unexpected roles in CD pathogenesis, independent of its transamidating and deamidating activity. For example, TG2 is easily targeted on the cell surface by anti-TG2 antibodies, and not only at the intestinal level. Given the biological consequences of such an interaction at the cell surface (for example, increased proliferation and structural and signalling modifications) and potential differences in subcellular TG2 distribution in CD cells, it is reasonable to conclude that TG2 may contribute to CD onset and progression in other peculiar and still unexplored fashions.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23147513
References
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell. Biol. 2003, 4,140–156.
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell. Biol. 2003, 4, 140–156.
- Stefania Martucciello; Silvia Sposito; Carla Esposito; Gaetana Paolella; Ivana Caputo; Interplay between Type 2 Transglutaminase (TG2), Gliadin Peptide 31-43 and Anti-TG2 Antibodies in Celiac Disease. International Journal of Molecular Sciences 2020, 21, 3673, 10.3390/ijms21103673.
- Rasmus Iversen; Sunniva F. Amundsen; Liv Kleppa; M. Fleur du Pré; Jorunn Stamnæs; Ludvig M. Sollid; Evidence That Pathogenic Transglutaminase 2 in Celiac Disease Derives From Enterocytes. Gastroenterology 2020, 159, 788-790, 10.1053/j.gastro.2020.04.018.
- Ludvig M. Sollid; Jason A. Tye-Din; Shuo-Wang Qiao; Robert P. Anderson; Carmen Gianfrani; Frits Koning; Update 2020: nomenclature and listing of celiac disease–relevant gluten epitopes recognized by CD4+ T cells. Immunogenetics 2019, 72, 85-88, 10.1007/s00251-019-01141-w.
- Ludvig M. Sollid; Jason A. Tye-Din; Shuo-Wang Qiao; Robert P. Anderson; Carmen Gianfrani; Frits Koning; Update 2020: nomenclature and listing of celiac disease–relevant gluten epitopes recognized by CD4+ T cells. Immunogenetics 2019, 72, 85-88, 10.1007/s00251-019-01141-w.
- L M Sollid; Ø Molberg; S McADAM; K E A Lundin; Autoantibodies in coeliac disease: tissue transglutaminase—guilt by association?. Gut 1997, 41, 851-852, 10.1136/gut.41.6.851.
- Barbara Lexhaller; Christina Ludwig; Katharina Anne Scherf; Identification of Isopeptides Between Human Tissue Transglutaminase and Wheat, Rye, and Barley Gluten Peptides.. Scientific Reports 2020, 10, 7426-14, 10.1038/s41598-020-64143-9.
- Jorunn Stamnæs; Rasmus Iversen; M. Fleur Du Pré; Xi Chen; Ludvig M. Sollid; Enhanced B-Cell Receptor Recognition of the Autoantigen Transglutaminase 2 by Efficient Catalytic Self-Multimerization. PLOS ONE 2015, 10, e0134922, 10.1371/journal.pone.0134922.
- Rasmus Iversen; M. Fleur Du Pré; Roberto Di Niro; Ludvig M. Sollid; Igs as Substrates for Transglutaminase 2: Implications for Autoantibody Production in Celiac Disease. The Journal of Immunology 2015, 195, 5159-5168, 10.4049/jimmunol.1501363.
- Rasmus Iversen; Bishnudeo Roy; Jorunn Stamnaes; Lene S. Høydahl; Kathrin Hnida; Ralf S. Neumann; Ilma R. Korponay-Szabó; Knut E. A. Lundin; Ludvig M. Sollid; Efficient T cell–B cell collaboration guides autoantibody epitope bias and onset of celiac disease. Proceedings of the National Academy of Sciences 2019, 116, 15134-15139, 10.1073/pnas.1901561116.
- Ludvig M Sollid; Bana Jabri; Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Current Opinion in Immunology 2011, 23, 732-738, 10.1016/j.coi.2011.08.006.
- Jan Petersen; Laura Ciacchi; Mai T. Tran; Khai Lee Loh; Yvonne Kooy-Winkelaar; Nathan P. Croft; Melinda Y. Hardy; Zhenjun Chen; James McCluskey; Robert P. Anderson; et al. T cell receptor cross-reactivity between gliadin and bacterial peptides in celiac disease. Nature Structural & Molecular Biology 2019, 27, 49-61, 10.1038/s41594-019-0353-4.
