Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Coronavirus disease 2019 (COVID-19) is a highly heterogeneous disease regarding severity, vulnerability to infection due to comorbidities, and treatment approaches. The hypothalamic–pituitary–adrenal (HPA) axis has been identified as one of the most critical endocrine targets of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that might significantly impact outcomes after infection.
- SARS-CoV-2
- glucocorticoids
- hypothalamic–pituitary–adrenal axis
- hypercortisolism
1. Introduction
COVID-19 is a highly heterogeneous disease regarding disease severity, vulnerability to infection due to underlying comorbidities, and administered medications. It has been demonstrated to be a systemic disease with effects extending beyond the respiratory system and often presents with protracted clinical manifestations lasting beyond the initial infection period [1][2]. The ubiquitous expression of angiotensin-converting enzyme 2 (ACE2), the primary receptor responsible for the entry of SARS-CoV-2 at the cellular level, combined with widespread endothelial damage and altered immune response, partially explain the multisystemic presentation of COVID-19 [1]. The involvement of the endocrine system in COVID-19 is so relevant that an “endocrine phenotype” of COVID-19 has progressively acquired clinical relevance [1]. However, while the contribution of endocrine dysfunction to the severity and outcomes of COVID-19 remains to be fully elucidated, the impact of SARS-CoV-2 on the endocrine system may be severely underreported due to the lack of awareness of the public and clinicians [2][3]. In particular, adrenal glands, with a crucial role in priming the immune system, might be a vulnerable and vital target during COVID-19, with direct and indirect effects on the overall outcomes. Furthermore, systemic corticosteroids remain the leading treatment choice in moderate and severe COVID-19 cases with acute and persistent effects on the hypothalamic–pituitary–adrenal (HPA) axis. In addition, pre-existing adrenal disorders may impact the susceptibility and severity of COVID-19, and special care is needed in the management of these patients during the current pandemic. Finally, with the long-term impact of COVID-19 presenting an increasing challenge for health care systems worldwide, the extent of the HPA axis’s contribution to this problem as a likely endocrine culprit remains a topic for future research.
2. The Impact of COVID-19 Infection on Hypothalamic–Pituitary–Adrenal Axis
Beyond GI-AI, several additional mechanisms can cause the observed impact of SARS-CoV-2 infection on the HPA axis. The various potential mechanisms by which SARS-CoV-2 can impair the HPA axis are presented in Figure 1.
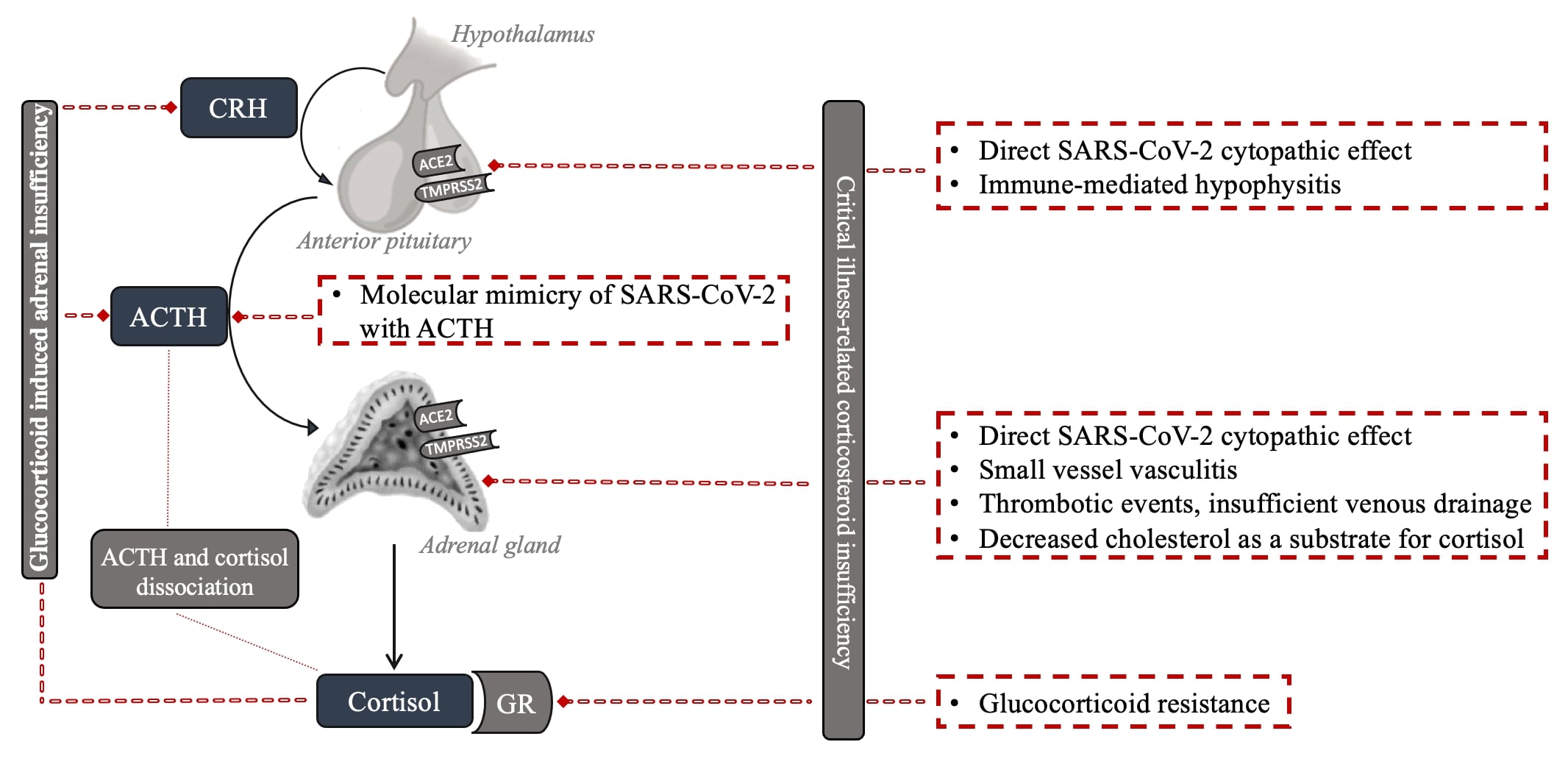
Figure 1: Potential mechanisms of HPA axis impairment with SARS-CoV-2 infection.
2.1. Critical Illness-Related Corticosteroid Insufficiency
Firstly, deregulation of the HPA axis may be encountered as part of the development of functional adrenal insufficiency (AI), as has been described in critical illness from 2000 onwards based on the initial work by Annane et al. in septic shock [4], later named critical illness-related corticosteroid insufficiency (CIRCI) [5]. This functional AI is hypothesized to be mainly due to inadequate cortisol secretion for the modulation of inflammatory responses in critically ill patients. Multiple mechanisms, including (i) reduced albumin or cortisol-binding globulins (CBGs), (ii) decreased affinity, (iii) reduced number of adrenal gland cortisol receptors, (iv) or increased activity of 11-β hydroxysteroid dehydrogenase type 2 resulting in cortisol inactivation, have been proposed in this regard [6]. In addition, higher levels of cytokines observed in patients with CIRCI could directly suppress ACTH release during critical illness because the pituitary gland is not protected by the blood–brain barrier [7]. New research performed over the last 10 years has led to the insight that the term CIRCI should be explicitly used for a condition that may develop in prolonged critically ill patients that are at risk of acquiring central adrenal insufficiency. In that setting, the adrenal cortex, depleted from ACTH-mediated trophic signalling for a prolonged period, may become structurally and functionally impaired, resulting in insufficient cortisol production [8]. Regardless of the potential mechanisms, severe COVID-19 cases are at potential risk of developing CIRCI. A small study reported CIRCI diagnosis in six out of nine critically ill patients with COVID-19 [9].
2.2. Adrenal Gland
The expression of two crucial viral receptors through which SARS-CoV-2 enters host cells, ACE2 and transmembrane protease serine 2 (TMPRSS2), have been documented in the adrenal zona fasciculata and reticularis of the adrenal gland [9][10]. Autopsy studies showed adrenal histomorphologic alterations that could be assigned to the SARS-CoV-2 infection, implying a direct cytopathic effect [11][12]. The adrenals showed mainly acute fibrinoid necrosis of small vessels, primarily affecting arterioles in the adrenal parenchyma, capsule, and periadrenal adipose tissue [13]. Additional findings included subendothelial vacuolization and apoptotic debris without significant signs of inflammation, parenchymal infarctions, or thrombosis. In another autoptic study of fatal COVID-19 cases, the authors identified SARS-CoV-2 and its replication in the adrenal glands, which co-localized with ACE2 and TMPRSS2, mainly in epithelial but also in mesenchymal and endothelial cells [10][14][15]. Some reports specifically observed chronic inflammation with perivascular distribution and vasculitis of the small vessels. The autopsy studies have involved a very limited number of patients so far and only included those more severely affected by multi-organ failure. Whether the histomorphologic alterations led to altered cortisol dynamics or induced insufficiency is questionable.
Furthermore, AI in COVID-19 patients may be due to thrombotic events [16]. Incidental adrenal computed tomography findings compatible with adrenal infarction have been reported in 23% of patients with severe COVID-19 [17], the vast majority with bilateral involvement. However, the clinically relevant cases are less frequently reported [18]. Of note, two out of nine clinically relevant cases had positive antiphospholipid antibodies prior to COVID-19 diagnosis, suggesting a previously described link between the antiphospholipid syndrome and the risk of bilateral adrenal infarction in non-COVID-19 patients as a potential mechanism [18][19][20][21].
The adrenal gland’s venous drainage might be another culprit [22]. The high stress from SARS-CoV-2 infection might be a limiting factor for a solitary suprarenal vein due to ACTH-induced arteriolar dilation, leading to vascular stasis and subsequent adrenal damage [23]. Moreover, decreased cholesterol, in the main form of high-density lipoprotein (HDL), as one of the main substrates for cortisol synthesis, might lead to hypocortisolism in severe COVID-19 infection [24].
2.3. Pituitary Gland and Hypothalamus
AI may also occur due to central dysfunction. An impaired adrenocortical response was reported in 28 patients with COVID-19 with plasma cortisol and ACTH concentrations indicating central hypocortisolism [25]. Several potential mechanisms have been hypothesized regarding the etiology of COVID-19-related central hypocortisolism. The expression of two receptors through which SARS-CoV-2 enters host cells, ACE2 and TMPRSS2, have also been documented in the hypothalamus and pituitary [26], making them possible direct cytopathic targets of SARS-CoV-2. In a postmortem study of COVID-19 patients, areas of necrosis/infarction were seen in one out of the nineteen pituitaries [27]. Genome sequences of SARS-CoV-2 have also been detected in the pituitary and hypothalamus postmortem studies, implying a direct hypothalamic injury induced by the virus [28][29]. Some authors also suggested a reversible immune-mediated hypophysitis [30].
A recent study in male mice established that the S1 subunit of the spike protein of SARS-CoV-2 crosses the blood–brain barrier and is taken up by brain regions that include the cortex, hypothalamus, and hippocampus, areas of particular importance for HPA axis control [31]. In small autoptic studies in patients infected with severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), the somatotroph, thyrotroph, and corticotroph pituitary cells’ number, as well as the respective hormone immunoreactivity, were reduced. However, the opposite was observed for mammotroph and gonadotroph pituitary cells [32]. Whether there are similar alterations with SARS-CoV-2 infection remains unknown.
Furthermore, it has been hypothesized that SARS-CoV-1 inhibits the adrenal stress responses causing a relative adrenocortical insufficiency via molecular mimicry of specific sequences of SARS-CoV-1 with ACTH and the immune response that cross-reacts with ACTH [28][33]. However, currently, there is no evidence to support this intriguing hypothesis. Another possibility might be related to cytokines, as in CIRCI. Extensive cytokine production (interleukin (IL)-1, IL-6, TNF-α, monocyte chemoattractant protein 1 (MCP1), and granulocyte-colony stimulating factor (G-CSF)) during COVID-19 infection reduces ACTH release and decreases its effect on adrenal tissue [34][35][36][37].
2.4. Resistance to Cortisol Action at the Level of Glucocorticoid Receptor and Postreceptor Signaling
The immunological, metabolic, and hemodynamic actions of glucocorticoids are mediated by a ubiquitous intracellular receptor, the glucocorticoid receptor (GR) [38]. The GR–cortisol complex translocates from the cytosol to the nucleus, where it exerts transcriptional activity, resulting in the inhibition of the inflammatory response. Preclinical septic models have reported glucocorticoid resistance in critical illness [38]. Glucocorticoid resistance may be a consequence of decreased GR mRNA and protein expression, reduced GR affinity for the ligand and nuclear translocation, and/or impaired DNA binding.
In patients with COVID-19, there is no evidence of resistance to cortisol action since the strong activation of an endogenous cortisol response to SARS-CoV-2 has been detected. It is, however, possible that the strong response is still insufficient to achieve maximum immunosuppression, providing grounds for corticotherapy in patients with COVID-19. In a recent study of single-cell RNA sequencing data, functionally active GR subunit mRNA expression from the bronchoalveolar lavage fluid was decreased in severe COVID-19 patients compared to mildly affected patients. The authors suggested that this might reflect a pathologic down-regulation of this endogenous immunomodulatory mechanism in patients with severe COVID-19 infection, which could be restored pharmacologically with corticosteroid therapy [39].
2.5. Dissociation between Cortisol and ACTH Regulation
Another phenomenon should be considered when interpreting the potential HPA axis deregulation in COVID-19 patients. ACTH and cortisol dissociation, attributed to the dependence of cortisol secretion from factors other than ACTH, has been described in severe illness. Cytokines, which are expected to be higher in the more severe illness, can stimulate cortisol secretion independently from ACTH. Reduced peripheral cortisol metabolism, resulting in increased systemic half-life, represents an additional ACTH-independent mechanism of increased cortisol levels with a consequent feedback reduction of ACTH [40]. In one study, ACTH levels tended to also be lower in those with moderate to severe COVID-19 with simultaneously increased cortisol levels, consistent with the published evidence of a dissociation between cortisol and ACTH levels in severe illness [41].
2.6. Clinical Data during Acute COVID-19 Infection
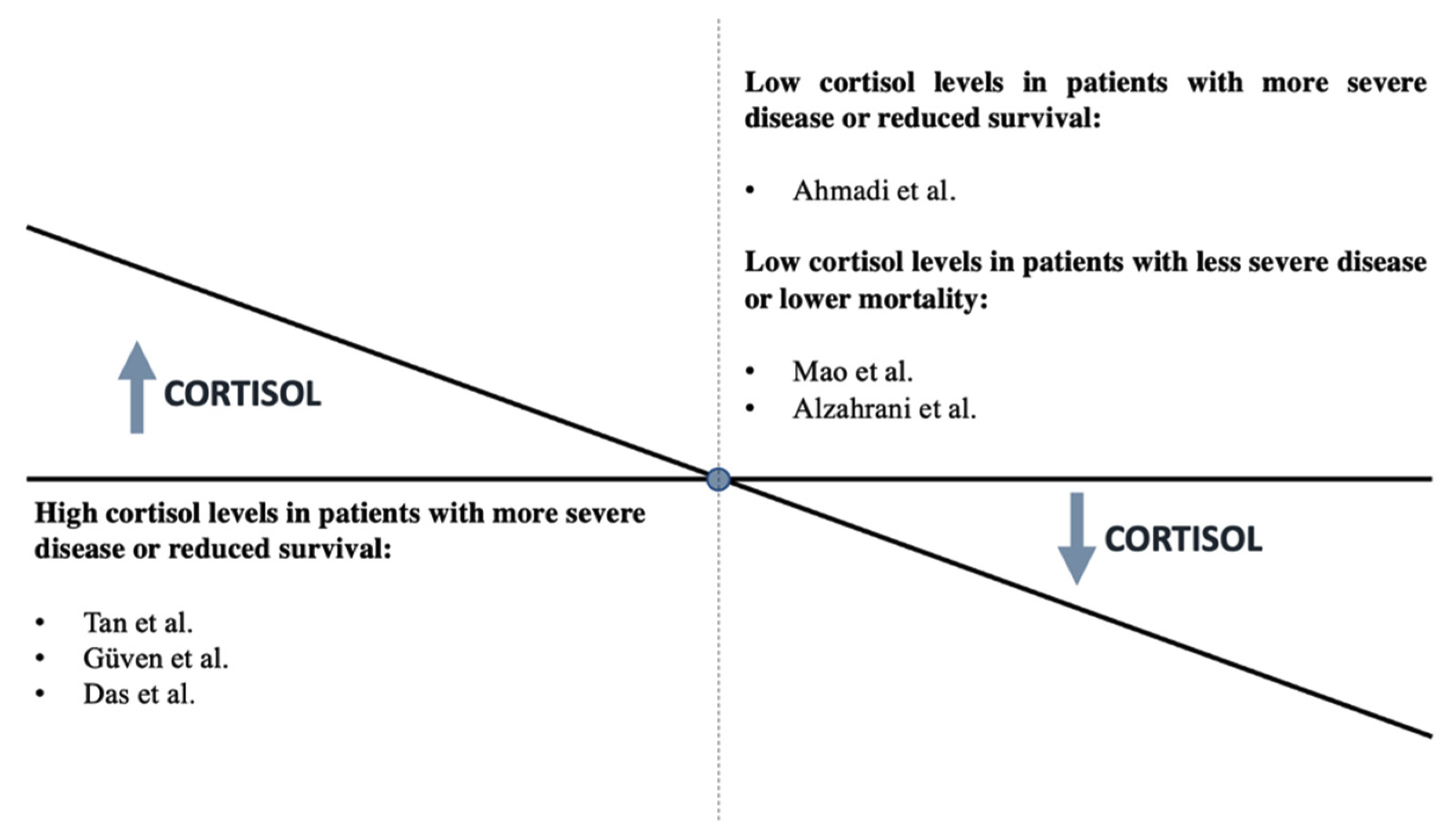
Beyond the above hypotheses, there are limited clinical data on HPA axis deregulation in COVID-19 patients during an acute infection. One limitation is the difficulty of performing a complete and exact evaluation of the HPA axis in patients with COVID-19 who are glucocorticoid dependent [42]. AI is a disease that is probably unrecognized in clinical settings during COVID-19. A systemic review of 10 studies that reported AI occurrence in patients who suffered from COVID-19 revealed the AI prevalence ranged from 3.1% to as high as 64.3%, indicating a high prevalence of AI in the COVID-19 pandemic without the clarification of the extent and type of AI [22]. Furthermore, some research groups evaluated the association of cortisol levels with the severity of COVID-19 disease, with conflicting outcomes.
In 403 non-critically ill COVID-19 patients, within 48 h of hospital admission, cortisol levels were significantly higher (619 (456–833) nmol/L) than in patients admitted to the hospital for other reasons (519 (378–684) nmol/L), indicating a marked and appropriate acute cortisol stress response (p < 0.0001) [43]. In a study of 144 COVID-19 critically ill patients, a multivariate logistic regression analysis demonstrated that mortality was associated with higher cortisol levels (odds ratio: 1.2; 95% confidence interval: 1.08–1.35; p = 0.001) and the cortisol cutoff point was 855 nmol/L for predicting mortality among COVID-19 patients [44]. A positive association with disease severity was also confirmed in a study that reported higher cortisol levels in COVID-19 patients with moderate to severe disease (433 (353–571) nmol/L) compared to those with mild disease (370 (279–454) nmol/L) (p = 0.053) [45]. A small study reported cortisol levels below 300 nmol/L (10 μg/dL) in a significant number of asymptomatic/mild cases, a finding that was not observed in repeated testing after a few days in most patients [25]. The researchers reported that doubling cortisol levels increased mortality by 42% [43].
By contrast, the most recent findings revealed that individuals with SARS-CoV-2 who had lower cortisol levels had a greater fatality rate [42]. A total of 154 hospitalized patients with COVID-19 were studied in a prospective cohort study. ACTH and cortisol levels in the blood were measured on the first or second day of hospitalization. Cortisol levels were substantially lower in those who died (311.7 (273.1–394.5) nmol/L) than in patients who were discharged (460.7 (344.8–598.6) nmol/L) (p = 0.003), while ACTH levels were unaffected. According to the logistic model, cortisol levels that rose by one unit correlated with a 26% lower mortality risk [42]. Overall, the existing evidence does not consistently support an association of severe COVID-19 with the presence of reduced or increased cortisol. Figure 2 summarizes the studies to date regarding the association between cortisol levels and disease severity. Further precise investigations with long-term follow-up are necessary.
2.7. Clinical Data in Patients Who Survive COVID-19 Infection
It is increasingly evident that the health impact of COVID-19 extends beyond the initial infection, with up to 63% of patients reporting ongoing symptoms [46]. Fatigue and some other symptoms could overlap with “post-COVID” or “long COVID” and AI. As AI is eminently treatable, it is imperative to identify any contribution it may have to the persistent symptoms experienced by patients after COVID-19 infection. A recently published study assessed the HPA axis in patients at least 3 months after diagnosis of COVID-19. They all had peak cortisol ≥450 nmol/L after tetracosactide, Synacthen (250 micrograms iv bolus), consistent with adequate adrenal reserve. Basal and peak serum cortisol did not differ according to disease severity or history of dexamethasone treatment (6 mg once daily for a maximum of 10 days during COVID-19). Patients who were prescribed other steroids (oral, inhaled, topical, or intra-articular) following recovery from COVID-19 and those taking other medications known to affect CBG (including oral estrogens) were excluded from the study. The authors reported for the first time that the fatigue after COVID-19 was not accounted for the overt adrenal dysfunction [46].
Another group analysed the persistence of symptoms and their impact on quality of life in people who had had COVID-19 one year after their admission for COVID-19, and they explored the influence of treatment with systemic corticosteroids during the acute phase of the illness. Most symptoms were less frequent in the group that received corticosteroids, with statistically significant differences for headache, dysphagia, chest pain, and depression. These patients also showed significantly better outcomes in the SF-36 domains for “bodily pain” and “mental health.” They concluded that corticosteroids administered in the acute phase of COVID-19 could attenuate the presence of long-term symptoms and improve patients’ quality of life [47].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23137326
References
- Puig-Domingo, M.; Marazuela, M.; Yildiz, B.O.; Giustina, A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine 2021, 72, 301–316.
- Clarke, S.A.; Abbara, A.; Dhillo, W.S. Impact of COVID-19 on the Endocrine System: A Mini-review. Endocrinology 2021, 163, bqab203.
- Mung, S.M.; Jude, E.B. Interplay between endocrinology, metabolism and COVID-19 infection. Clin. Med. 2021, 21, e499–e504.
- Annane, D.; Sébille, V.; Troché, G.; Raphaël, J.C.; Gajdos, P.; Bellissant, E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000, 283, 1038–1045.
- Annane, D.; Pastores, S.M.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; Marik, P.E.; et al. Critical illness-related corticosteroid insufficiency (CIRCI): A narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 2017, 43, 1781–1792.
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017, 43, 1751–1763.
- Ferraù, F.; Ceccato, F.; Cannavò, S.; Scaroni, C. What we have to know about corticosteroids use during Sars-Cov-2 infection. J. Endocrinol. Investig. 2021, 44, 693–701.
- Téblick, A.; Gunst, J.; van den Berghe, G. Critical Illness-induced Corticosteroid Insufficiency: What It Is Not and What It Could Be. J. Clin. Endocrinol. Metab. 2022, 107, 2057–2064. Available online: https://pubmed.ncbi.nlm.nih.gov/35358303 (accessed on 2 April 2022).
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front. Endocrinol 2020, 11, 593179.
- Wong, D.W.L.; Klinkhammer, B.M.; Djudjaj, S.; Villwock, S.; Timm, M.C.; Buhl, E.M.; Wucherpfennig, S.; Cacchi, C.; Braunschweig, T.; Knuchel-Clarke, R. Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19. Patients Cells 2021, 10, 1900.
- Santana, M.F.; Borba, M.G.S.; Baía-Da-Silva, D.C.; Val, F.; Alexandre, M.A.A.; Brito-Sousa, J.D.; Melo, G.C.; Queiroga, M.V.O.; Farias, M.E.L.; Camilo, C.C.; et al. Case Report: Adrenal Pathology Findings in Severe COVID-19: An Autopsy Study. Am. J. Trop Med. Hyg. 2020, 103, 1604–1607.
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253.
- Iuga, A.C.; Marboe, C.C.; Yilmaz, M.; Lefkowitch, J.H.; Gauran, C.; Lagana, S.M. Adrenal Vascular Changes in COVID-19 Autopsies. Arch. Pathol. Lab. Med. 2020, 144, 1159–1160.
- Salzano, C.; Saracino, G.; Cardillo, G. Possible Adrenal Involvement in Long COVID Syndrome. Medicina 2021, 57, 1087.
- Piticchio, T.; le Moli, R.; Tumino, D.; Frasca, F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic—A comprehensive review. J. Endocrinol. Investig. 2021, 44, 1553–1570.
- Bellastella, G.; Maiorino, M.I.; Esposito, K. Endocrine complications of COVID-19: What happens to the thyroid and adrenal glands? J. Endocrinol. Investig. 2020, 43, 1169–1170.
- Leyendecker, P.; Ritter, S.; Riou, M.; Wackenthaler, A.; Meziani, F.; Roy, C.; Ohana, M. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: A retrospective cohort analysis on 219 patients. Eur. Radiol. 2021, 31, 895–900.
- Machado, I.F.R.; Menezes, I.Q.; Figueiredo, S.R.; Coelho, F.M.A.; Terrabuio, D.R.B.; Ramos, D.V.; Fagundes, G.; Maciel, A.; Latronico, A.; Fragoso, M. Primary adrenal insufficiency due to bilateral adrenal infarction in COVID-19: A case report. J. Clin. Endocrinol. Metab. 2021, dgab557.
- Espinosa, G.; Santos, E.; Cervera, R.; Piette, J.-C.; de la Red, G.; Gil, V.; Font, J.; Couch, R.; Ingelmo, M.; Asherson, R. Adrenal involvement in the antiphospholipid syndrome: Clinical and immunologic characteristics of 86 patients. Medicine 2003, 82, 106–118.
- Presotto, F.; Fornasini, F.; Betterle, C.; Federspil, G.; Rossato, M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: Report of a case and review of the literature. Eur. J. Endocrinol. 2005, 153, 507–514.
- Ramon, I.; Mathian, A.; Bachelot, A.; Hervier, B.; Haroche, J.; Huong, D.B.-L.T.; Costedoat-Chalumeau, N.; Wechsler, B.; Karmali, R.; Velkeniers, B.; et al. Primary Adrenal Insufficiency Due to Bilateral Adrenal Hemorrhage-Adrenal Infarction in the Antiphospholipid Syndrome: Long-Term Outcome of 16 Patients. J. Clin. Endocrinol. Metab. 2013, 98, 3179–3189.
- Vakhshoori, M.; Heidarpour, M.; Bondariyan, N.; Sadeghpour, N.; Mousavi, Z. Adrenal Insufficiency in Coronavirus Disease 2019 (COVID-19)-Infected Patients without Preexisting Adrenal Diseases: A Systematic Literature Review. Int. J. Endocrinol. 2021, 2021, 2271514.
- Piccioli, A.; Chini, G.; Mannelli, M.; Serio, M. Bilateral massive adrenal hemorrhage due to sepsis: Report of two cases. J. Endocrinol. Investig. 1994, 17, 821–824.
- Chien, J.-Y.; Jerng, J.-S.; Yu, C.-J.; Yang, P.-C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005, 33, 1688–1693.
- Alzahrani, A.S.; Mukhtar, N.; Aljomaiah, A.; Aljamei, H.; Bakhsh, A.; Alsudani, N.; Elsayed, N.; Alrashidi, N.; Fadel, R.; Alsudani, N. The Impact of COVID-19 Viral Infection on the Hypothalamic-Pituitary-Adrenal Axis. Endocr Pr. Off. J. Am. Coll Endocrinol Am. Assoc. Clin. Endocrinol. 2021, 27, 83–89.
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031.
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; AlRasheed, R. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod. Pathol Off. J. United States Can. Acad Pathol Inc. 2021, 34, 1456–1467.
- Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 2020, 68, 251–252.
- Siejka, A.; Barabutis, N. Adrenal insufficiency in the COVID-19 era. Am. J. Physiol. 2021, 320, E784–E785.
- Leow, M.K.-S.; Kwek, D.S.-K.; Ng, A.W.-K.; Ong, K.-C.; Kaw, G.J.-L.; Lee, L.S.-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin. Endocrinol. 2005, 63, 197–202.
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.; Raber, J.; Banks, W.; Erickson, M. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378.
- Wei, L.; Sun, S.; Zhang, J.; Zhu, H.; Xu, Y.; Ma, Q.; McNutt, M.A.M.A.; Korteweg, C.; Gu, J. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem. Cell Biol. 2010, 88, 723–730.
- Wheatland, R. Molecular mimicry of ACTH in SARS—Implications for corticosteroid treatment and prophylaxis. Med. Hypotheses 2004, 63, 855–862.
- Bateman, A.; Singh, A.; Kral, T.; Solomon, S. The immune-hypothalamic-pituitary-adrenal axis. Endocr. Rev. 1989, 10, 92–112.
- Soni, A.; Pepper, G.M.; Wyrwinski, P.M.; Ramirez, N.E.; Simon, R.; Pina, T.; Gruenspan, H.; Vaca, C.E. Adrenal insufficiency occurring during septic shock: Incidence, outcome, and relationship to peripheral cytokine levels. Am. J. Med. 1995, 98, 266–271.
- Natarajan, R.; Ploszaj, S.; Horton, R.; Nadler, J. Tumor necrosis factor and interleukin-1 are potent inhibitors of angiotensin-II-induced aldosterone synthesis. Endocrinology 1989, 125, 3084–3089.
- Guarner, J.; Paddock, C.D.; Bartlett, J.; Zaki, S.R. Adrenal gland hemorrhage in patients with fatal bacterial infections. Mod. Pathol. 2008, 21, 1113–1120.
- Vassiliadi, D.A.; Vassiliou, A.G.; Ilias, I.; Tsagarakis, S.; Kotanidou, A.; Dimopoulou, I. Pituitary—Adrenal Responses and Glucocorticoid Receptor Expression in Critically Ill Patients with COVID-19. Int. J. Mol. Sci. 2021, 22, 11473.
- Awasthi, S.; Wagner, T.; Venkatakrishnan, A.J.; Puranik, A.; Hurchik, M.; Agarwal, V.; Conrad, I.; Kirkup, C.; Arunachalam, R.; O’Horo, J.; et al. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021, 7, 55.
- Van den Berghe, G.; Boonen, E.; Walker, B.R. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 2013, 369, 481.
- Bornstein, S.R.; Engeland, W.C.; Ehrhart-Bornstein, M.; Herman, J.P. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 2008, 19, 175–180.
- Ahmadi, I.; Estabraghnia Babaki, H.; Maleki, M.; Jarineshin, H.; Kaffashian, M.R.; Hassaniazad, M.; Kenarkoohi, A.; Ghanbarnejad, A.; Falahi, S.; Jahromi, M.; et al. Changes in Physiological Levels of Cortisol and Adrenocorticotropic Hormone upon Hospitalization Can Predict SARS-CoV-2 Mortality: A Cohort Study. Int. J. Endocrinol. 2022, 2022, 4280691.
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660.
- Güven, M.; Gültekin, H. Could serum total cortisol level at admission predict mortality due to coronavirus disease 2019 in the intensive care unit? A prospective study. Sao Paulo Med. J. 2021, 139, 398–404.
- Das, L.; Dutta, P.; Walia, R.; Mukherjee, S.; Suri, V.; Puri, G.D.; Mahajan, V.; Malhotra, P.; Chaudhary, S.; Gupta, R.; et al. Spectrum of Endocrine Dysfunction and Association with Disease Severity in Patients with COVID-19: Insights From a Cross-Sectional, Observational Study. Front. Endocrinol. 2021, 12, 645787.
- Clarke, S.; Phylactou, M.; Patel, B.; Mills, E.G.; Muzi, B.; Izzi-Engbeaya, C.; Choudhury, S.; Khoo, B.; Meeran, K.; Comninos, A.N.; et al. Normal Adrenal and Thyroid Function in Patients Who Survive COVID-19 Infection. J. Clin. Endocrinol. Metab. 2021, 106, 2208–2220.
- Catalán, I.P.; Martí, C.R.; de la Sota, D.P.; Álvarez, A.C.; Gimeno, M.J.E.; Juana, S.F.; Rodríguez, G.H.; Bajo, E.D.; Gaya, N.T.; Blasco, J.U.; et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J. Med. Virol. 2022, 94, 205–210.
This entry is offline, you can click here to edit this entry!
