Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Epstein-Barr virus (EBV), the representative of the Herpesviridae family, is a pathogen extensively distributed in the human population. One of its most characteristic features is the capability to establish latent infection in the host. The infected cells serve as a sanctuary for the dormant virus, and therefore their desensitization to apoptotic stimuli is part of the viral strategy for long-term survival.
- Epstein-Barr virus
- apoptosis
1. Introduction
Apoptosis, a type I cell death, plays an essential role in the development of the organism and is necessary for its proper function. The aforementioned process normally happens during organ morphogenesis as a mechanism responsible for the controlled elimination of unwanted cells [1]. Apoptosis may be inscribed in scenario of natural growing and aging, but it also occurs in response to a wide range of incidental factors and conditions, e.g., high temperature [2,3], UV radiation [4,5,6] as well as an exposition to alcohol (ethanol) [7,8] or toxic metal ions [9,10].
Apoptosis is also the biological phenomenon that contributes to defense against intracellular infectious agents, both bacteria and viruses. Programmed cell death frustrates intracellular replication of pathogens, counteracting the development of disease. Therefore, bacteria and viruses have evolved molecular mechanisms to inhibit apoptosis and maintain the environment of their effective multiplication [11,12,13,14,15,16,17]. Research revealed anti-apoptotic activity of intracellular bacteria, Chlamydia trachomatis, [11] as well as of a wide range of viruses, such as vaccinia virus (VACV) [16,17], hepatitis B virus (HBV) [15,18], hepatitis C virus (HCV) [12,14], Kaposi’s sarcoma-associated herpesvirus (KSHV) [19] and Epstein–Barr virus (EBV) [13]. The last two pathogens, γ-herpesviruses, are causative agents of lytic and persistent infections that may lead to the development of several diseases including malignancies [19,20].
2. EBV and Its Pathogenicity
Epstein–Barr virus (EBV), a member of the Herpesviridae family and γ-Herpesvirinae subfamily, is a virus widely distributed in the human population. A total of 95% of people worldwide are estimated to carry EBV. The virion contains a 170–175 kb linear dsDNA genome [21] enclosed in an icosahedral capsid of 100–120 nm in diameter. The nucleocapsid is surrounded by a tegument and encircled by a lipid envelope decorated with glycoproteins. Viral DNA, comprising approximately 90 reading frames [22], is flanked by terminal direct repeats (TRs) and divided into short and long unique sequence domains by internal repeat sequences (IRs) [23]. EBV replicates in B, T, and natural killer (NK) lymphocytes as well as in epithelial cells [21,24]. The virus is characterized by the ability to establish persistent infection. The life cycle of EBV includes primary infection followed by latency and lytic replication [21,25]. The adaptations of EBV to establish latent infection are associated with the oncogenic properties of this pathogen [26].
EBV may be carried by healthy individuals without any symptoms of its presence. However, immunosuppression accompanying acquired immune deficiency syndrome (AIDS) or transplantation treatment favors EBV-driven fatal lymphoproliferative disease [21,25]. Moreover, EBV may contribute to the development of several malignancies, such as B cell lymphomas (i.e., Burkitt’s lymphoma (BL) [27,28,29,30,31], Hodgkin’s lymphoma (HL) [32,33], and diffuse large B-cell lymphoma (DLBCL) [34,35,36]), lung carcinoma (LC) [37,38,39], gastric cancer (GC) [40,41,42], nasopharyngeal carcinoma (NPC) [43,44,45], T cell lymphoma [46], T/NK cell lymphoma (NKTL) [47,48], and AIDS-related primary central nervous system lymphoma (AR-PCNSL) [49]. EBV has also been detected in the saliva of patients with systemic lupus erythematosus (SLE), and found to be associated with one of its symptoms (i.e., oral lesions) [50]. In BL development, the viral products complement with a cellular protein named c-myelocytomatosis oncogene product (c-Myc). The disease results from the deregulation of c-Myc expression following the translocation of its gene into immunoglobulin (Ig) loci. EBV synergizes with c-Myc in pathogenic activity by preventing apoptosis of the mutant cell [51].
The virus encodes proteins that promote the progression of EBV-associated diseases. Some of them, BamH1 fragment H rightward facing (BHRF1), BamH1-A reading frame-1 (BARF1), latent membrane proteins (LMP)-1 and -2A, EBV nuclear antigen (EBNA)-1, -2, -leader protein (LP), -3A and -3C, inhibits apoptosis of infected cells. Research has also revealed the anti-apoptotic role of EBV-encoded small RNAs (EBERs) and microRNAs (miRNAs) of EBV (Figure 1). Therefore, treatment strategies discussed in medical literature include therapies aimed at inducing apoptosis in infected cells [52,53,54].
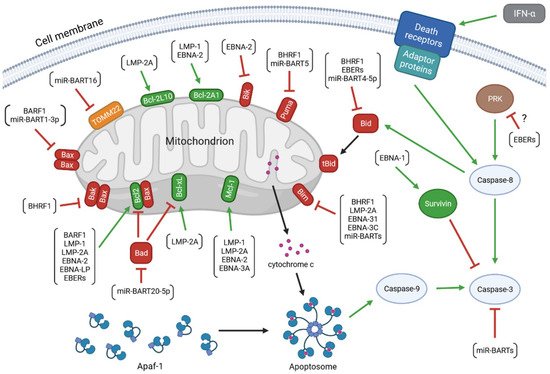
Figure 1. The anti-apoptotic activity of the EBV-encoded products. The colors of arrows represent the impact of the upstream molecules on the expression and/or activity of the target proteins (green: up-regulation; red: down-regulation). The molecular network determining the viability of the infected cell includes: (1) viral proteins: BamH1 fragment H rightward facing (BHRF1), BamH1-A reading frame-1 (BARF1), latent membrane proteins (LMP)-1 and -2A, EBV nuclear antigen (EBNA)-1, -2, -leader protein (LP), -3A and -3C; (2) viral non-coding transcripts: EBV-encoded small RNAs (EBERs), BamHI A rightward transcripts (miR-BARTs), and miR-BHRF1s; (3) anti-apoptotic members of the B-cell lymphoma 2 (Bcl-2) protein family: Bcl-2, B-cell lymphoma-extra large protein (Bcl-xL), myeloid leukemia cell differentiation protein 1 (Mcl-1), Bcl-2-like protein 10 (Bcl-2L10), and Bcl-2-related protein A1 (Bcl-2A1); (4) pro-apoptotic members of the Bcl-2 family: Bcl-2-associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak), Bcl-2-associated agonist of cell death (Bad), Bcl-2-like protein 11 (Bim), BH3-interacting domain death agonist (Bid), p53 up-regulated modulator of apoptosis (Puma), and Bcl-2 interacting killer (Bik); (5) other cellular molecules: mitochondrial import receptor subunit TOM22 homolog (TOMM22), apoptotic protease activating factor 1 (Apaf-1), cytochrome c, caspases(-8, -9, and -3), survivin, interferon (IFN)-α, death receptors, adaptor proteins, and dsRNA-dependent protein kinase (PKR).
EBV infection may lead to the host cell transformation, a process associated with changes in the levels of several viral proteins: BHRF1, EBNA-1, -2, -Leader Protein (LP), -3A, -3B, 3C and LMP-1, -2A, -2B. The EBV products include also non-coding RNAs: EBERs and microRNAs (miRNAs) including BamHI A rightward transcripts (miR-BARTs) and miR-BHRF1 molecules [13]. There are a few infection patterns with different viral gene expression profiles (Figure 2). One of them, the Latency I pattern, is restricted to the expression of EBNA-1, EBERs, and miR-BARTs. The small set of EBV products implicates limited anti-apoptotic effect of EBV infection. Latency I program occurs in BL tumor cells. Another expression pattern, Latency II, is characterized by the expression of three LMPs (LMP-1, -2A, and -2B) and the products present in Latency I. The program of Latency II is realized in HL, NPC, and NKTL. The exclusive expression of BARF1 has also been detected in the NPC cells displaying this program. Next, a latent viral promoter Wp-restricted latency includes synthesis of BHRF1, EBNA-1, -LP, -3A, -3B, -3C, EBERs, and miR-BARTs. This pattern is characteristic of some BL tumor cells. Another program, Latency III, is distinguished by the expression of the full range of proteins and non-coding RNAs (BHRF1, EBNA-1, -2, -LP, -3A, -3B, 3C, LMP-1, -2A, -2B, EBERs, miR-BARTs, and miR-BHRF1s), and observed in vitro in resting B-lymphocytes converting into lymphoblastoid cell lines (LCLs). This pattern occurs also in DLBCL [13,42,55,56,57,58,59,60].
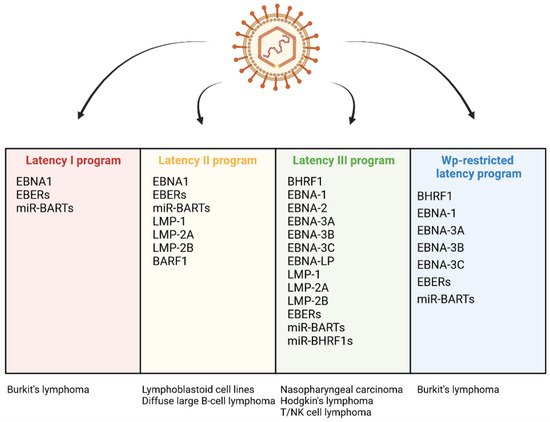
Figure 2. Differential expression programs in distinct types of EBV latency and various EBV-associated diseases.
In some cases, the establishment of latency may be followed by a switch to a productive cycle aimed at the formation and release of progeny virions [61,62]. The lytic cycle consists of three stages: the immediate-early (IE) phase, the early one, and the late one. In the first phase, the IE proteins are involved in the transcription of the viral genetic material. Next, the products of the early genes act to mediate DNA replication. In the third stage of the lytic cycle, the late proteins are engaged in the formation of progeny virions. The complete productive cycle ends in the infected cell lysis [63]. The switch between the latent period and the lytic cycle depends on the expression of the genes encoding two IE products, BamHI Z EBV replication activator (ZEBRA/BZLF1) and BRLF1 [62,64]. These genes are inactive during the latent infection, whereas upon the lytic phase, they undergo activation. ZEBRA and BRLF1 play the role of transcription factors responsible for the induction of the expression of the early genes encoding a set of lytic proteins, including BHRF1, BARF1, DNA primase (BSLF1), mRNA export factor ICP27 homolog (BMLF1/BSLF2), major DNA-binding proteins (BALF)2 and 5, primase-associated factors (BBLF)2/3 and 4, DNA polymerase processivity factor (BMRF1), thymidine kinase (TK), uracil-DNA glycosylase (BKRF3) and tegument protein (BKRF4) [62,65,66,67,68]. The replication of viral DNA, occurring in the early phase of the lytic cycle, is followed by the production of the late proteins (i.e., structural molecules such as major tegument protein (BNRF1), viral capsid antigen (VCA), major capsid protein (MCP), envelope glycoprotein gp350 (BLLF1), and envelope glycoprotein B (gB)). The progeny virions are assembled from the late viral products. Interestingly, the viral particles leave the nucleus and the cell, acquiring and losing sequentially the envelopes derived from the inner nuclear membrane and Golgi apparatus, respectively. The release of the virions to the extracellular environment occurs via the fusion of the organelle-derived envelope with the plasma membrane [62,66].
The lytic cycle leads to the multiplication of the virus and its effective spreading between the host cells. As a way of the carcinogenic pathogen propagation, it may favor the EBV-associated oncogenesis, contributing to the progression of both lymphoid and epithelial malignancies. Moreover, the individual viral lytic proteins may perform various oncogenic activities. They have been found to induce the genomic aberration, promote the expression and secretion of pro-inflammatory cytokines, counteract NK cell and CD8+ T cell response, increase angiogenesis and tumor invasiveness, as well as down-regulate major histocompatibility complexes (MHCs) and suppress MHC class II-restricted antigen presentation [63].
BHRF1 and BARF1 are present in the infected cells in some cases of latency, but also during the lytic cycle. The anti-apoptotic activity of these proteins increases the viability of the cell and delays its death in order to preserve the environment of replication and enable the completion of the progeny virions formation [62,69]. BHRF1 and BARF1 are synthesized in the early phase of the lytic cycle. BHRF1 gene is also expressed during the transient pre-latent abortive lytic cycle, a phase that precedes the latent period of infection [62,70].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23137265
This entry is offline, you can click here to edit this entry!
