Multiple sclerosis (MS) is defined as an immune-mediated inflammatory, neurodegenerative, and demyelinating disease that impacts the central nervous system (CNS) in young individuals. Although there are controversies regarding the exact mechanism of disease initiation, it is well known that inflammation plays an important role in pathogenesis of the disease. As important players in inflammation, cytokines, and adipokines, the effects of post-exercise on these factors in MS patients are still in the early stages of research, and results are in doubt. Due to the above differences, this study was undertaken to comprehensively evaluate randomized clinical trials (RCTs) examining the effects of exercise on inflammatory markers that have shown moderate to large changes in PwMS.
- multiple sclerosis
- inflammatory markers
- demyelinating autoimmune diseases
1. Introduction
IL-10 after exercise [11][16][22][23]. Two studies indicated that drawing clear conclusions about the impact of training on cytokine (ILs and TNF-α) and adipokine (leptin) levels in MS patients is impossible, and that exercise had no effect on MS clinical manifestations of systemic inflammation [11][24]. The effects of post-exercise cytokines and adipokines on MS patients are still in the early stages of research, and results are in doubt. Due to the above differences in the effect of exercise on cytokines and adipokines, this study was undertaken to comprehensively evaluate randomized clinical trials (RCTs) examining the effects of exercise on inflammatory markers that have shown moderate to large changes in PwMS. In the hopes of acquiring insight into the effects of cytokines and adipokines on the pathogenesis of MS, as well as the role of exercise, finding an alternative complementary treatment for these patients is a priority. In addition, the present research provides a systematic review of the effect of exercise on PwMS as a secondary aim of mental and physical health.
2. Materials and Methods
2.1. Literature Search Strategy
A search was independently conducted through electronic databases including Scopus, Web of Science, The Cochrane Library, and PubMed by two researchers to find studies addressing the effects of physical activity and/or exercise training on serum levels of inflammatory markers in PwMS. The search language was restricted to English and Persian. The search date was limited to studies that were published from January 2003 to April 2022. This systematic review was conducted using specific keywords such as “multiple sclerosis” AND (“exercise” OR “yoga” OR “physical endurance” OR “exercise movement techniques” OR “resistance training”) AND (“interleukins” OR “tumor necrosis factor-alpha” OR “cytokines” OR “inflammation” OR “interferons” OR “adipokines” OR “leptin” OR “chemokines”). The two reviewers conducted the searches independently and prescreened the initial stage of the study selection, including the analysis of titles and abstracts. In the second stage, the full-text studies were evaluated to select them according to the eligibility criteria. A third author was responsible for supervising the procedure and resolving any discrepancies.
2.3. Eligibility Criteria
All RCTs that evaluated the effect of any exercise or physical activity on the serum level of inflammatory markers in MS patients were included. Cytokines, chemokines, and adipokines were among the inflammatory markers evaluated in the blood and CSF in this research. Studies included had to satisfy the following criteria: (1) RCTs if they were well described and of high quality, with defined outcomes; (2) studies on MS patients who engage in regular physical activity; (3) research on proinflammatory and anti-inflammatory cytokines or adipokines. Moreover, studies with the following criteria were excluded from the current review: articles not in English or Persian, nonhuman trials, interventions besides supplements and medicines, absence of full-text studies, duplicate reports, reviews, studies, comments, opinion pieces, methodological reports, and conference abstracts. Figure 1 depicts the screening process.
2.4. Study Selection and Data Extraction
Data from the studies were independently collected and recorded in a Microsoft Excel database by two reviewers. Again, these processes were supervised by a third researcher. Variables extracted included the first author’s name, gender, sample size, age, disease status, Expanded Disability Status Scale (EDSS), type of exercise, duration and frequency of exercise, evaluated cytokines, a secondary outcome, type of sampling, and final results of each paper.
3. Results
In total, 7529 papers were found; 3490 duplicates and 4039 articles that did not fulfill the inclusion criteria were removed, leaving 1046 papers for abstract and title screening. Following a full-text review, 22 articles were chosen for further analysis. The PRISMA flowchart shows a summary of the search and research selection process (Figure 1).
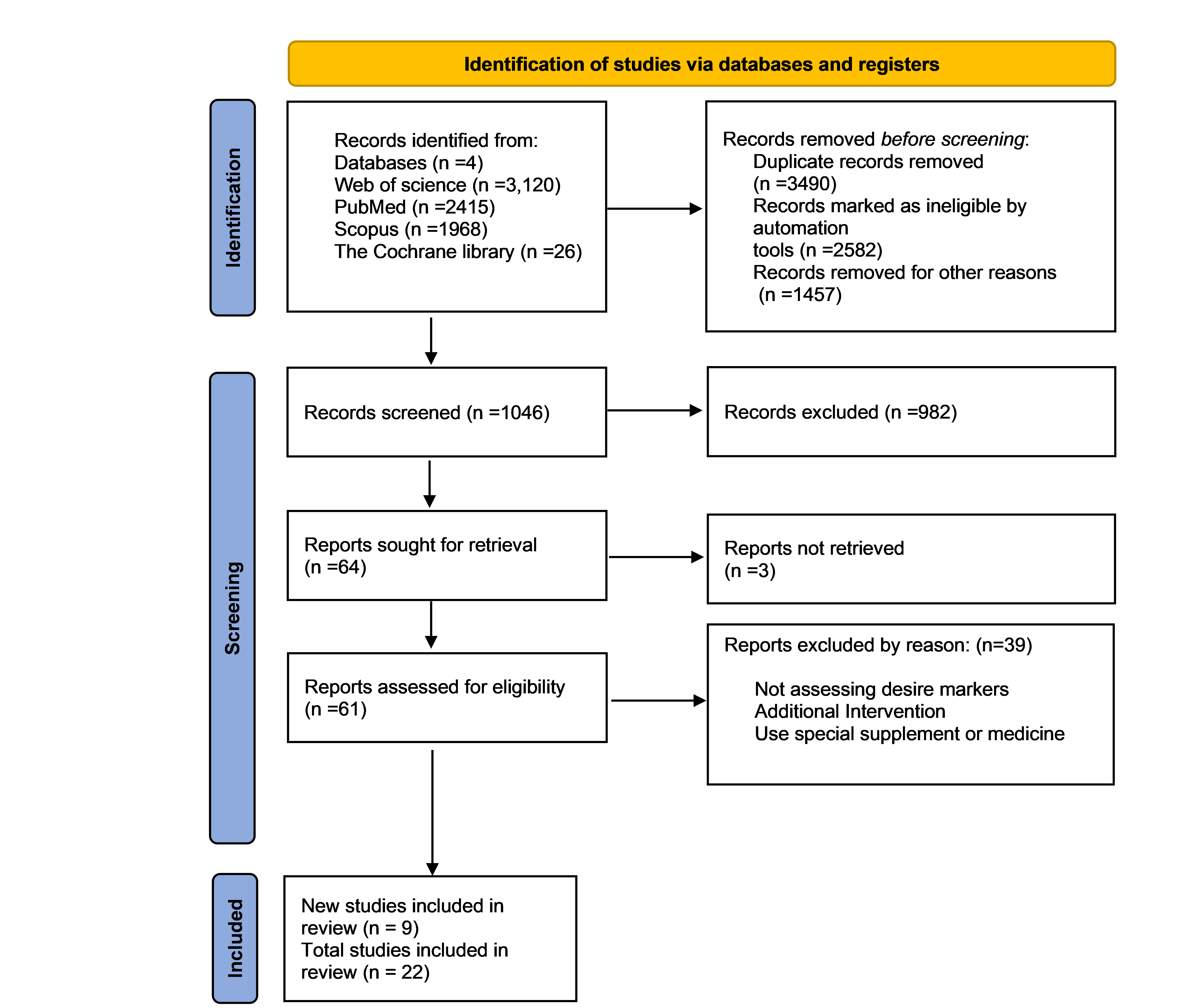
Figure1:PRISMA flow diagram study selection and inclusion process
3.1. Classification of Evidence
3.1.1. IL-6
Interleukin-6 was the most commonly assessed inflammatory marker, which was reported in 14 out of 22 studies[20][22][23][25][26][27][28][29][30][31][32][33][34][35]. The majority of the research showed no considerable changes in IL-6 levels, including five studies after using ergometers [22][26][27][28][30], two studies with combined training [25][34], one study after aerobic training [23], and another study with resistance exercise [29]. Three studies reported a decrease after 8 and 12 weeks (t.i.w.) of combined training [31][33] and 12 weeks of resistance exercise (b.i.w.) [32]. Only two studies evaluated high levels of IL-6 after one session of fitness [20] and 8 weeks (b.i.w.) of cycle ergometer exercises[35].
3.1.2. TNF-α
TNF-α was studied in 11 of 22 trials [16][22][23][25][26][28][29][34][36][37][38], with the majority of studies showing no significant difference in TNF-α levels with varying exercises from one session for 24 weeks [23][26][29][34][36][37]; 8 weeks of aerobic (t.i.w.) exercise [16], 12 weeks (t.i.w.) of combined training [25], and one session of ergometer training [22] resulted in lower serum TNF-α levels. Only one study reported an increase in serum TNF-α level following 8 weeks (t.i.w.) of cycle ergometer exercises [28].
3.1.3. IFN-γ
Eight studies reported IFN-γ serum levels; however, three studies reported no significant changes after three treadmill sessions [23], 12 weeks (t.i.w.) of combined exercise [25], and 24 weeks (b.i.w.) of resistance exercises [38]. Moreover, three studies evaluated a lower level of IFN-γ after 8 weeks of combined training [37][38][39] and 8 weeks of resistance exercises [29]. In contrast, in two other studies, IFN-γ was increased after 8 weeks of ergometer exercises [28] and 12 weeks of combined exercise [31].
3.1.4. IL-12 and IL-12p7
0Two studies reported a decrease in post-exercise serum levels (8 to 12 weeks, 2 to 3 days a week) following resistance and aqua training, respectively [32][40]. Only one study reported no significant changes after combined training [34], while another reported no significant changes after 8 weeks of ergometer exercises in serum levels of IL-12p70 [35].
3.1.5. IL-10
Eleven trials assessed IL-10 levels, and no noticeable changes in IL-10 levels were detected after performing combined exercises in three studies [25][34][37], and after resistance [36], Pilates [47], and aerobic exercises [16] in one study each. Although four studies reported a significant reduction in IL-10 level after resistance training [35], ergometer exercises [22][35], and three sessions of aerobic exercises [23], one research showed an enhanced IL-10 level after 8 weeks (t.i.w.) of combination training [33].
3.1.6. IL-4
Two trials found no significant differences after 8 weeks (t.i.w.) of combined training [41], three treadmill sessions [23], and 24 weeks of resistance exercise [36]; White et al. and Kierkegaard et al. found a decline in serum IL-4 levels after 8 and 12 weeks (b.i.w.) resistance training, respectively.
3.1.7. Adipokines
Three trials examined leptin following exercise, and two of them also assessed adiponectin. Two of them reported a decline in leptin serum levels after one session on an ergometer [22] and 8 weeks of aerobic training [16]. Only Ebrahimi et al. reported no considerable difference after 10 weeks (t.i.w.) of WBV. Furthermore, two studies following aerobic training [16] and one session of ergometer training [22] observed an increase and no changes in adiponectin serum levels, respectively.
3.1.8. BDNF
Three studies from five trials [20][26][27][30][47] reported a boost in BDNF serum levels after 3 and 9 (b & t.i.w.) weeks of cycle ergometry [26][27], and 8 weeks of Pilates training [47]. BDNF serum levels remained unchanged in two studies with ergometer exercises and fitness interventions [20][30].
3.2. Physical and Mental as a Secondary Outcome
Functional muscle strength [18][29][30][32][36][39] and fatigue [16][18][26][29][30][32] were the most examined factors, as evidenced by six articles, the majority of which showed better muscle function, and half of which reported fatigue treatments. Five trials reported an improvement in QoL and walking function after the period of intervention [18][22][30][31][32][35][36]. .
Table 2. Sample characteristics and main findings of the reviewed studies.
|
First Author |
Gender |
Sample Size |
Mean Age |
Disease Status |
Mean EDSS |
Type of Exercise |
Duration and Frequency of Exercise |
Evaluated Cytokines |
Main Findings |
|
Tadayon Zadeh F |
Female |
MST:15 MSC:15 |
Range (25–40) |
- |
≤6 |
Endurance, resistance, balance |
8 wks (t.i.w.), 40–70% HR max |
IL-6, CRP, IL-10 |
↓: IL-6, CRP ↑: IL-10 |
|
Devasahayam. A |
Both |
MST:14 MSC:8 |
54.07 (8.46) |
SPMS PPMS |
6–6.5 |
Fitness |
One session |
BDNF IL-6 |
↔: BDNF ↑: IL-6 |
|
Faramarzi M. |
Female |
MST:46 MSC:43 |
Range (18–50) |
RRMS |
Not reported range (0–8) |
Combined stretching, balance, pilates, resistance, endurance |
12 wks (t.i.w.) |
IL-6, IFN-γ, CRP |
↓:IL-6, CRP ↑: IFN-γ |
|
Rezaee S. |
Both |
MST:10 MSC:10 |
28.9 ± 3.3 |
RRMS |
2.2 ± 0.4 |
Aerobic training |
6 wks (t.i.w.) 60% VO2 max |
TNF- α |
↓: TNF- α |
|
Nejatpour S |
Male |
MST:13 MSC:12 |
- |
- |
Range (2.5 –5) |
Aqua training |
8 wks (t.i.w.) 75% VO2 max |
IL-12, Il-17 |
↓:IL-12, IL-17 |
|
Barry A |
Both |
MST:9 HC:10 |
35.33 ± 2.12 |
RRMS |
2.17 ± 0.40 |
Cycle ergometer |
8 wks (b.i.w.), 65–75% VO2 max |
IL-10, IL-12p70, IL-6 |
↑: IL-6, 12 p70 ↓: IL-10 |
|
Berkowitz, S. |
Female |
MST:15 MSC:10 |
33.8 ± 7.8 |
- |
1.5 |
Aerobic (treadmill) 50–80 VO2 max |
3 sessions |
IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNF-α |
↓: IL-10 ↔: IL-4, IL-17A, IFN-γ, TNF-α, IL-6 |
|
Eftekhari E. |
Female |
MST:15 MSC:15 |
34.46 ± 7.29 |
RRMS |
Range (2–6) |
Pilates training |
8 wks (t.i.w.) |
IL-10, BDNF |
↔: IL-10 ↑: BDNF |
|
Majidnasab N. |
Female |
MST:30 HC:15 MSC:5 |
28.23 ± 3.65 |
RRMS |
2.11 ± 0.76 |
Arm, cycle ergometer |
One session 60–70% VO2 max |
IL-6, IL-10, TNF- α, leptin, adiponectin |
↔: IL-6, adiponectin ↓: TNF- α, IL-10, leptin |
|
Mokhtarzade M. |
Female |
MST:22 MSC:8 |
32.04 ± 2.81 |
RRMS |
1.84 ± 0.35 |
Aerobic |
8 wks (t.i.w.) 60% max watt |
IL-10 TNF- α Leptin adiponectin |
↔: IL-10 ↓: leptin, TNF- α ↑: adiponectin |
|
Alvarenga-Filho H |
Both |
MST:8 MSC:10 HC:10 |
41.1 ± 12.9 |
RRMS |
0–2.5 |
Resistance training, cycle ergometer, pilates |
12 wks (t.i.w.) |
IL-6, IL-10, IL-21, IL-22, IL-17, TNF-α, IFN-γ |
↓:IL-22 ↔: IL-17, -10, -21, TNF- α, IL-6, IFN-γ |
|
Briken S. |
Both |
MST:28 MSC:9 |
49.9 ± 7.6 |
PPMS, SPMS |
4.9 ± 0.9 |
Endurance, arm ergometer, cycle ergometer, rowing |
9 wks (b & t.i.w.) |
BDNF, IL-6 |
↔: IL-6 ↑: BDNF |
|
Kierkegaard M. |
Both |
MST:20 |
36.3 ± 7.6 |
RRMS |
1.5 |
Resistance training |
12 wks (b.i.w.) 80% 1 RM |
IL-1ra, -4, -5, -6, -7, -8, -12p70, -13, -17 |
↓: all in blood ↔: all in CSF |
|
Deckx N. |
Both |
MST:29 MSC:16 |
47 ± 2 |
RRMS and CPMS |
3 ± 0.2 |
Endurance, resistance |
12 wks (t.i.w.) |
IL-6, IL-10, IL-12p70, TNF- α |
↔: all |
|
Ebrahimi.A |
Both |
MST:16 MSC:14 |
38.76 ± 9.66 |
RRMS |
3.11 ± 0.99 |
WBV |
10 wks (t.iw.) |
leptin |
↔: leptin |
|
Kjølhede T. |
Both |
MST:16 MSC:16 |
44.6 ± 7 |
RRMS |
2.9 ± 1 |
Progressive resistance training |
24 wks (b.i.w.) |
IL-1β, IL-4, IL-10, IL-17F, IL-23, TNF-α, IFN-γ |
↔: all |
|
Bansi J. |
Both |
WT:24 LT:28 |
44.6–56.3 |
- |
4.65 |
Cycle ergometer, aquatic bike |
3 wks, 70% H-peak |
BDNF, TNF- α, IL-6, sIL-6r |
↑: BDNF ↔: NGF, TNF-α, IL-6, sIL-6r |
|
Golzari Z. |
Female |
MST:10 MSC:10 |
32.15 ± 7.57 |
- |
2.14 ± 1.06 |
Stretch, aerobic, resistance, endurance |
8 wks (t.i.w.) |
IFN-γ, IL-4, IL-17 |
↓: IFN-γ, IL-17 ↔: IL-4 |
|
Castellano V. |
Both |
MST:11 MSC:11 |
40 ± 10 |
- |
0–5.5 |
Cycle ergometer |
8 wks (t.i.w.) 60% VO2 max |
TNF- α, IL-6, IFN-γ |
↔: IL-6 ↑: TNF- α, IFN-γ |
|
White L.J. |
Female |
MST:11 |
47 ± 12 |
- |
3.8 ± 0.9 |
Resistance training |
8 wks (b.i.w.) 50–70% MVC |
IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, CRP |
↓:IL-4, IL-10, IFN-γ, IL-2, CRP ↔: IL-6, TNF- α |
|
Schulz K. |
Both |
MST:15 MSC:13 |
42 ± 9.5 |
RRMS, SPMS |
2.5 ± 1.4 |
Cycle ergometer |
8 wks (b.i.w.) 75% VO2 max |
BDNF, NGF, IL-6, sIL-6r |
↔: All |
|
Heesen C. |
Both |
MST:15 MSC:13 HC:20 |
39.8 |
RRMS, SPMS, PPMS |
2.3 ± 0.2 |
Cycle ergometer (resistance + endurance) |
8 wks (b.i.w.) 60% VO2 max |
IFN-γ, TNF- α, IL-10 |
↓: IFN-γ ↔: TNF- α, IL-10 |
Weeks = wks; three times a week= (t.i.w.); two times a week = (b.i.w.); two and three times a week= (b & t.i.w.), not reported = (-), MS = multiple sclerosis; IL = interleukin; TNF-α = tumor necrosis factor-alpha; RCT = randomized controlled trial; RRMS = relapsing-remitting multiple sclerosis; PPMS = primary progressive multiple sclerosis; CNS: central nervous system ; SPMS = secondary progressive multiple sclerosis; CPMS = chronic progressive multiple sclerosis; BDNF = brain-derived neurotrophic factor ; NGF = nerve growth factor; CRP = C-reactive protein; IFN-ɣ = interferon-γ; EDSS = Expanded Disability Status Scale; IL-1Ra = IL-1 receptor antagonist; Th = T-helper; ↓:increased; ↑:decreased; ↔: no significant changes; MST: MS training group; MSC: MS control group; HC: healthy control group; WT: water training group; LT: land training group.
4. discussion
4.1. Proinflammatory Cytokines
4.2. Anti-Inflammatory Cytokines
4.3. Adipokines
4.4. BDNF
4.5. Physical and Mental Health as a Secondary Outcome
5. Conclusions
The current review did not provide a consensus on the effects of different exercise training protocols on the serum level of inflammatory markers in patients with MS. This may be attributed to variations in the population gender, design and duration of studies, and inflammatory marker measurement protocols. Although it was indicated that acute exercise induces short-term inflammation followed by a mid-term anti-inflammatory environment, there are still many unanswered questions about the beneficial methodological flaws in the face of inflammatory factors.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19138151
References
- Rodríguez Murúa, S.; Farez, M.F.; Quintana, F.J. The immune response in multiple sclerosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 121–139.
- Najafi, P.; Moghadasi, M. The effect of yoga training on enhancement of Adrenocorticotropic hormone (ACTH) and cortisol levels in female patients with multiple sclerosis. Complement. Ther. Clin. Pract. 2017, 26, 21–25.
- Valadkeviciene, D.; Kavaliunas, A.; Kizlaitiene, R.; Jocys, M.; Jatuzis, D. Incidence rate and sex ratio in multiple sclerosis in Lithuania. Brain Behav. 2019, 9, e01150.
- Schreiner, B.; Becher, B. Perspectives on cytokine-directed therapies in multiple sclerosis. Swiss Med. Wkly. 2015, 145, w14199.
- Alfredsson, L.; Olsson, T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944.
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273.
- Bai, Z.; Chen, D.; Wang, L.; Zhao, Y.; Liu, T.; Yu, Y.; Yan, T.; Cheng, Y. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: A systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front. Neurosci. 2019, 13, 1026.
- ‘t Hart, B.A.; Luchicchi, A.; Schenk, G.J.; Stys, P.K.; Geurts, J.J. Mechanistic underpinning of an inside–out concept for autoimmunity in multiple sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 1709–1719.
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558.
- Friese, M.A.; Schattling, B.; Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2014, 10, 225.
- Wong, V.L.; Holahan, M.R. A systematic review of aerobic and resistance exercise and inflammatory markers in people with multiple sclerosis. Behav. Pharmacol. 2019, 30, 652–659.
- Patel, D.I.; White, L.J. Effect of 10-day forced treadmill training on neurotrophic factors in experimental autoimmune encephalomyelitis. Appl. Physiol. Nutr. Metab. 2013, 38, 194–199.
- Bettcher, B.M.; Johnson, S.C.; Fitch, R.; Casaletto, K.; Heffernan, K.S.; Asthana, S.; Zetterberg, H.; Blennow, K.; Carlsson, C.M.; Neuhaus, J. CSF and plasma levels of inflammation differentially relate to cns markers of alzheimer’s disease pathology and neuronal damage. Alzheimer’s Dement. 2017, 13, P689.
- Alexander, W.S. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2002, 2, 410–416.
- Döring, A.; Pfueller, C.F.; Paul, F.; Dörr, J. Exercise in multiple sclerosis--an integral component of disease management. Epma J. 2012, 3, 2.
- Mokhtarzade, M.; Ranjbar, R.; Majdinasab, N.; Patel, D.; Molanouri Shamsi, M. Effect of aerobic interval training on serum IL-10, TNFα, and adipokines levels in women with multiple sclerosis: Possible relations with fatigue and quality of life. Endocrine 2017, 57, 262–271.
- Gondim, O.S.; de Camargo, V.T.N.; Gutierrez, F.A.; de Oliveira Martins, P.F.; Passos, M.E.P.; Momesso, C.M.; Santos, V.C.; Gorjão,R.; Pithon-Curi, T.C.; Cury-Boaventura, M.F. Benefits of regular exercise on inflammatory and cardiovascular risk markers in normal weight, overweight and obese adults. PLoS ONE 2015, 10, e0140596.
- Ebrahimi, A.; Eftekhari, E.; Etemadifar, M. Effects of whole body vibration on hormonal & functional indices in patients with multiple sclerosis. Indian J. Med. Res. 2015, 142, 450.
- Gjevestad, G.O.; Holven, K.B.; Ulven, S.M. Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: A systematic review. Curr. Cardiovasc. Risk Rep. 2015, 9, 34. [CrossRef] [PubMed]
- Devasahayam, A.J.J.; Kelly, L.P.P.; Williams, J.B.B.; Moore, C.S.; Ploughman, M. Fitness shifts the balance of BDNF and IL-6 from inflammation to repair among people with progressive multiple sclerosis. Biomolecules 2021, 11, 504. [CrossRef]
- Mee-Inta, O.; Zhao, Z.-W.; Kuo, Y.-M. Physical exercise inhibits inflammation and microglial activation. Cells 2019, 8, 691.[CrossRef] [PubMed]
- Majdinasab, N.; Motl, R.W.; Mokhtarzade, M.; Zimmer, P.; Ranjbar, R.; Keytsman, C.; Cullen, T.; Negaresh, R.; Baker, J.S. Acute responses of cytokines and adipokines to aerobic exercise in relapsing vs. remitting women with multiple sclerosis. Complement. Ther. Clin. Pract. 2018, 31, 295–301.
- Berkowitz, S.; Achiron, A.; Gurevich, M.; Sonis, P.; Kalron, A. Acute effects of aerobic intensities on the cytokine response in women with mild multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 31, 82–86.
- Negaresh, R.; Motl, R.W.; Mokhtarzade, M.; Dalgas, U.; Patel, D.; Shamsi, M.M.; Majdinasab, N.; Ranjbar, R.; Zimmer, P.; Baker, J.S. Effects of exercise training on cytokines and adipokines in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2018, 24, 91–100.
- Alvarenga, H.; Sacramento, P.M.; Ferreira, T.B.; Hygino, J.; Abreu, J.E.C.; Carvalho, S.R.; Wing, A.C.; Alvarenga, R.M.P.; Bento, C.A.M. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J. Neuroimmunol. 2016, 293, 91–99.
- Bansi, J.; Bloch, W.; Gamper, U.; Kesselring, J. Training in MS: Influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Mult. Scler. J. 2013, 19, 613–621.
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.H.; Gold, S.M. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016, 299, 53–58.
- Castellano, V.; Patel, D.I.; White, L.J. Cytokine responses to acute and chronic exercise in multiple sclerosis. J. Appl. Physiol. 2008, 104, 1697–1702.
- White, L.J.; Castellano, V.; McCoy, S.C. Cytokine responses to resistance training in people with multiple sclerosis. J. Sports Sci. 2006, 24, 911–914.
- Schulz, K.-H.; Gold, S.M.; Witte, J.; Bartsch, K.; Lang, U.E.; Hellweg, R.; Reer, R.; Braumann, K.-M.; Heesen, C. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J. Neurol. Sci. 2004, 225, 11–18.
- Faramarzi, M.; Banitalebi, E.; Raisi, Z.; Samieyan, M.; Saberi, Z.; Ghahfarrokhi, M.M.; Negaresh, R.; Motl, R.W. Effect of combined exercise training on pentraxins and pro- inflammatory cytokines in people with multiple sclerosis as a function of disability status. Cytokine 2020, 134, 9.
- Kierkegaard, M.; Lundberg, I.E.; Olsson, T.; Johansson, S.; Ygberg, S.; Opava, C.; Holmqvist, L.W.; Piehl, F. High-intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J. Neurol. Sci. 2016, 362, 251–257.
- Zadeh, F.T.; Amini, H.; Habibi, S.; Shahedi, V.; Isanejad, A.; Akbarpour, M. The Effects of 8-Week Combined Exercise Training on Inflammatory Markers in Women with Multiple Sclerosis. Neurodegener. Dis. 2020, 20, 212–216.
- Deckx, N.; Wens, I.; Nuyts, A.H.; Hens, N.; De Winter, B.Y.; Koppen, G.; Goossens, H.; Van Damme, P.; Berneman, Z.N.; Eijnde, B.O.; et al. 12 weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediat. Inflamm. 2016, 2016, 6789276.
- Barry, A.; Cronin, O.; Ryan, A.M.; Sweeney, B.; O’Toole, O.; O’Halloran, K.D.; Downer, E.J. Cycle ergometer training enhances plasma interleukin-10 in multiple sclerosis. Neurol. Sci. 2019, 40, 1933–1936.
- Kjølhede, T.; Dalgas, U.; Gade, A.; Bjerre, M.; Stenager, E.; Petersen, T.; Vissing, K. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand. J. Med. Sci. Sports 2016, 26, 824–834.
- Heesen, C.; Gold, S.M.; Hartmann, S.; Mladek, M.; Reer, R.; Braumann, K.M.; Wiedemann, K.; Schulz, K.H. Endocrine and cytokine responses to standardized physical stress in multiple sclerosis. Brain Behav. Immun. 2003, 17, 473–481.
- Rezaee, S.; Kahrizi, S.; Nabavi, S.M.; Hedayati, M. Vegf and tnf-α responses to acute and chronic aerobic exercise in the patients with multiple sclerosis. Asian J. Sports Med. 2020, 11, 98312. [CrossRef]
- Golzari, Z.; Shabkhiz, F.; Soudi, S.; Kordi, M.R.; Hashemi, S.M. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int. Immunopharmacol. 2010, 10, 1415–1419.
- Nejatpour, S.; Fathei, M.; Yaghoubi, A. The effect of aqua-therapy on plasma and interleukin-12 and 17 in patients with multiple sclerosis. Sport Tk-Rev. Euroam. Cienc. Deport. 2019, 8, 89–93. [CrossRef]
- Bergmann, M.; Gornikiewicz, A.; Sautner, T.; Waldmann, E.; Weber, T.; Mittlböck, M.; Roth, E.; Függer, R. Attenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsis. Shock 1999, 12, 421–427.
- Barbado, D.; Gomez-Illan, R.; Moreno-Navarro, P.; Mendoza, N.; Vaillo, R.R.; Sempere, A.P. Does exercise have a neuroprotective function in multiple sclerosis? A brief overview of the physical training potential effects on cytokines and brain-derived neurotrophic factor. Eur. J. Hum. Mov. 2018, 41, 124–148.
- Jensen, J.; Krakauer, M.; Sellebjerg, F. Cytokines and adhesion molecules in multiple sclerosis patients treated with interferon-β1b. Cytokine 2005, 29, 24–30.
- Sharief, M. Cytokines in multiple sclerosis: Pro-inflammation or pro-remyelination? Mult. Scler. J. 1998, 4, 169–173.
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142.
- Podbielska, M.; O’Keeffe, J.; Pokryszko-Dragan, A. New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7319.
- Eftekhari, E.; Etemadifar, M. Interleukin-10 and brain-derived neurotrophic factor responses to the Mat Pilates training in women with multiple sclerosis. Sci. Med. 2018, 28, 31668.
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64.
- Wong, V.L.; Holahan, M.R. A systematic review of aerobic and resistance exercise and inflammatory markers in people with multiple sclerosis. Behav. Pharmacol. 2019, 30, 652–659. [CrossRef] [PubMed]
- Nejatpour, S.; Fathei, M.; Yaghoubi, A. The effect of aqua-therapy on plasma and interleukin-12 and 17 in patients with multiple sclerosis. Sport Tk-Rev. Euroam. Cienc. Deport. 2019, 8, 89–93. [CrossRef]
