Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
液晶弹性体(LCE)是可编程的可变形材料,可以响应物理场,如光、热和电。光热驱动的LCE具有精度和远程控制的优点,避免了光化学对高光子能量的要求。光热驱动LCEs材料不可或缺的部分是光热材料。
- liquid crystal elastomer
- smart material
- photothermal-driven liquid crystal elastomers
1. Liquid Crystal Elastomers
Since Finkelmann et al. first reported liquid crystal elastomers in 1981, liquid crystal elastomers (LCEs) have under-gone tremendous development in both material chemistry and processing, becoming a class of materials with special properties [11]. Unlike liquid crystal polymers that are not crosslinked and liquid crystal networks that are highly crosslinked, low-crosslinked liquid crystal polymer networks (Figure 1a) with anisotropic polymer chains are known as LCEs [4]. Due to the low degree of crosslinking, anisotropic polymer chains can temporarily become isotropic when heated beyond the nematic-to-isotropic phase transition temperature (TNI), resulting in contraction along the alignment (Figure 1b). At the same time, the degree of order in the LCEs is reduced with the loss of anisotropic chain conformation. The presence of the polymer network ensures that the LCE can obtain the original orientation when the temperature is lower than TNI. This determines that the LCE undergoes reversible anisotropic deformation when the temperature changes. In fact, different application scenarios require LCEs that possess different orientations and physical properties, which are affected by the synthesis and alignment of LCEs. In general, the synthetic routes can be summarized into two groups: two-step crosslinking and one-step crosslinking. For the two-step cross-linking route, the LCE can be mechanically oriented because of the presence of a partially cross-linked polymer network. In the one-step crosslinking route, low molecular weight monomers are directly polymerized to form LCEs. Due to the low viscosity of the precursors, the LCEs prepared by one-step cross-linking are suitable for surface-induced orientation.
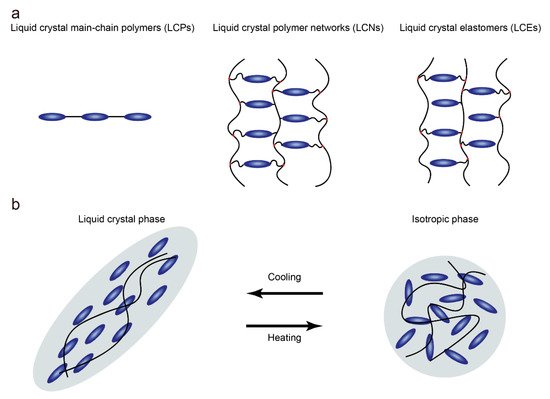
Figure 1. (a) Schematic of liquid crystal polymers, liquid crystal polymer networks and liquid crystal elastomers; (b) Schematic illustration of the contraction along the alignment of LCEs when heated beyond TNI.
1.1. Two-Step Cross-Linking
The two-step crosslinking method proceeds in two steps, the first step forming a weakly crosslinked, partially reacted polymer network, whose shape and orientation can be easily changed. Through mechanical stretching and other methods, monodomain LCEs with the desired shape can be obtained, and the shape and orientation are fixed by the second-step reaction. A typical example of two-step cross-linking was presented by Finkelmann et al., which was based on liquid crystal polysiloxanes [11]. In the presence of a platinum catalyst, linear polysiloxane chains are mixed and reacted with vinyl liquid crystal monomers and multifunctional vinyl crosslinkers (Figure 2a). Taking advantage of the difference in the reaction rates of the two different cross-linking agents, the first-step reaction produces weakly cross-linked polymers, and aligns the polymer chains by mechanical stretching. Under the condition of weight loading, the second part of the polymerization reaction forms a strong cross-linked polymer network and permanently maintains a uniform director orientation.
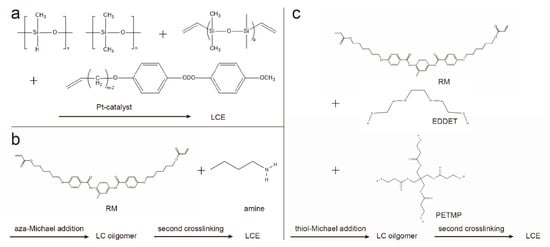
Figure 2. Synthetic route for two-step cross-linked LCE chemistry. (a) Two-step crosslinking by hydrosilylation; (b) aza-Michael addition between diacrylate-based RMs and amines; (c) thiol-Michael addition of diacrylate-based RMs and thiols.
The two-step hydrosilylation reaction route relies on non-commercially available materials. Therefore, the preparation of LCEs based on the chain extension reaction of diacrylate-based reactive mesogens (RMs) is gaining popularity, including the aza-Michael addition method [3,12] and thiol-Michael addition method [13]. Because both of the above mentioned methods are based on purchasable liquid crystal monomers and other components, the initiation conditions of the two-step polymerization are orthogonal based on the chain extension reaction, enabling easy access and good controllability. For the aza-Michael addition (Figure 2b), a classic example is based on the chain extension of liquid crystal diacrylate monomers via aza-Michael addition with amines [3,14], where the low viscosity of the reactive precursor and the slow reaction rate can be used for surface alignment [3,15]. The thiol-Michael addition reaction can be applied to chain extension to form oligomers (Figure 2c). After the alignment of oligomers, the orientation of the mesogens is then fixed by photopolymerization [16,17].
1.2. One-Step Cross-Linking
Low molar mass liquid crystals have been studied for many years and are frequently used in daily life due to optical anisotropy and electrical tunability. Alignment techniques for low molar mass liquid crystals are well developed [18,19], which can also be used to align low-viscosity precursors of LCEs. Compared with the two-step cross-linking method, the low molar mass liquid crystal monomers are directly polymerized to form LCE in the one-step cross-linking method without the process of chain growth and partial cross-linking, which results in better maintenance of orientation. It is worth noting that this alignment technique is usually based on a treated surface, which possesses strong programmability, but the anchoring force weakens with distance. Due to the limitation of the anchoring force, LCEs fabricated by this alignment method are thin, and the LCE precursor is required to have a low viscosity. In the one-step cross-linking method, the polymerizable liquid crystal monomer, cross-linking agent, and initiator are directly mixed. Since no pre-polymerization process is required and the viscosity of the precursor is low, alignment can be induced by a surface to achieve spatially complex orientation. The first example of a one-step cross-linked LCE was synthesized by free-radical polymerization of acrylates [2]. Thomsen et al. used acrylate-functionalized LC monomers for crosslinking to obtain side-chain LCEs. Orientation was based on surface-induced orientation, where the material was poured into glass cells coated with a rubbed polyvinyl alcohol film, then cooled from the isotropic phase (95 °C) to the nematic phase (85 °C) at −1 °C/min to obtain a good orientation. This method only takes a few minutes to obtain LCEs, which saves time compared to two-step crosslinking.
2. LCE-Based Composite Material
光热驱动LCEs材料不可或缺的部分是光热材料。光热材料可以吸收特定波长的光,将光子能转化为热能,并将热量传递给LCE进行驱动。某些种类的光热材料的引入也可以增强LCE的机械性能。光热转化材料的研究范围从无机到有机,包括碳基材料、金属纳米材料和有机染料。
碳基材料 (碳纳米管、石墨烯、GO)
碳基材料通常是市售的,通常具有优异的导热性和机械性能。碳纳米管(CNTs)是由石墨烯片轧制而成的圆柱形管。圆柱体的直径一般为纳米,长度在几微米的范围内[20]。碳纳米管具有将发光能转化为热能的能力,并表现出很高的光热转换效率,因此它们是LCE中常用的光热转换材料[21,22]。早在2003年,Courty等人[23]就报道了CNT和LCE的复合。当时,由于纳米管和聚合物的不相容性,碳纳米管的质量比非常低,小于0.02重量%,这使得它们难以在高浓度下均匀地分布在LCE基质中。为了获得更高的光热转化效率和力学性能,提高碳纳米管浓度已成为一个重要命题。2008年,Yang等人[24]在不改变其固有性质的情况下改变了碳纳米管的表面性质,实现了单壁碳纳米管(SWCNTs)在LCE基体中的高分散性,并实现了近红外光驱动的可逆变形。2010年,Ji等人[21]制备了一种芘封端的LCE,以促进碳纳米管的分散,然后将LCE拉伸以诱导CNT与LCE的取向对齐,从而获得分散良好且排列均匀的CNT。基于LCE-CNT复合材料的优异光机械性能,它们在各个领域具有应用的潜力。例如,已经报道了人造光驱动的盲文显示器[25]。得益于碳纳米管较强的光热转换效率,制造了一种太阳驱动的向日葵状致动器,可有效提高太阳能电池板的工作效率[26]。LCE-CNT复合材料也应用于制造轻质软机器人,以实现爬行,挤压和跳跃的多模态运动[27]。
作为最受欢迎的材料之一,石墨烯也被纳入LCE基复合材料中。与碳纳米管一样,石墨烯在近红外区域表现出良好的光热性能。Yang等人[28]报道,通过在LCE中排列石墨烯片,石墨烯片可以充当纳米加热器并触发LCE的相变,从而实现高达35.7%的光驱动宏观变形。石墨烯具有自聚集的倾向,导致复合材料的不均匀性。相比之下,氧化石墨烯(GO)的边缘含有含氧基团和羰基和羧基,因此易于改性和溶解具有吸引力。Li等人[29]制备了GO-LCE纳米复合薄膜,其表现出强大的光热机械响应,单轴收缩率达到33%,有效载荷驱动增加约50%。
金属奈米材料 (AuNP, AuNR)
光热金属纳米材料,如金和银,当入射光子频率与金属纳米颗粒的整体振动频率相匹配时产生强烈的吸收,产生大量的热量。这是一种由局部表面等离子体共振(LSPR)产生的现象。强大的光热效应为光热驱动的LCE提供了一种新的方法。除了光热效应外,Montazami等人[31]还发现将金纳米颗粒(AuNPs)嵌入LCE中可以提高LCE的导热性,杨氏模量和响应速度。在这项研究中,响应速度可以提高100%以上。Sun等人[32]浸润了微米级的LCE圆柱形致动器,其金纳米晶直径约为2纳米。通过光学镊子实现圆柱形微执行器的空间平移,对准和旋转,并通过聚焦近红外(NIR)激光束激发圆柱形微执行器的弯曲变形。Liu等人[30]通过在LCE单体中直接分散金纳米球(AuNS)和金纳米棒(AuNR)来探索可见光照射下的光热驱动LCE。通过紫外光固化获得LCE /AuNS和LCE/AuNR复合材料,在635 nm激光照射下实现了30%的驱动应变。Xu等人[33]利用5纳米金纳米颗粒制备了金纳米颗粒/LCE纳米复合材料.金纳米粒子的吸收带约为475 nm至近625 nm,占据了太阳光谱的主要部分,从而产生了可以由阳光驱动的光热驱动的LCE。然而,金纳米颗粒也存在团聚问题。Wójcik等人[34]使用纳米颗粒作为LCE材料的交联剂,在LCE基体中实现均匀分散,并确保LCE的良好取向。
有机材料 (有机染料,PDA)
与碳基复合材料和金属纳米颗粒相比,有机材料更容易与LCE相互作用。聚多巴胺(PDA)因其在近红外区域具有出色的光稳定性和强大的光热效应而受到越来越多的关注。Tian等人[35]使用PDA涂覆LCE薄膜以制造光学响应的LCE薄膜。通过巧妙地利用PDA的特性,可以同时在大气和水环境中工作,制造出一种仿生鱼,可以在近红外光下在水面上游泳。Lan等人[36]利用聚多巴胺涂层的可重写特性来制造选择性涂有PDA的LCE振荡器。PDA强大的光热转换能力使LCE能够被阳光驱动,实现了将太阳能转化为电能的功能。除PDA外,分散红等有机染料已广泛应用于LCE光热致动器和软机器人[14,15,17,27,37,38]。
This entry is adapted from the peer-reviewed paper 10.3390/molecules27144330
This entry is offline, you can click here to edit this entry!
