Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
The development of lithium-ion batteries largely relies on the cathode and anode materials. In particular, the optimization of cathode materials plays an extremely important role in improving the performance of lithium-ion batteries, such as specific capacity or cycling stability. Carbon coating modifying the surface of cathode materials is regarded as an effective strategy that meets the demand of Lithium-ion battery cathodes.
- lithium-ion battery
- carbon coating
- specific capacity
1. Introduction
Lithium-ion batteries (LIB) are well known as the most promising candidate in the electrochemical energy storage system and power source, due to the excellent features of light weight, high power and energy density, high current discharge, and long service lifetime [1,2]. It is considered by scientists and governments because of the extremely broad application in the portable consumer electronics and automobile market [3]. To meet the requirement of energy storage, decreasing the energy consumption [4,5] and developing the next generation, LIB becomes increasingly important [6,7].
Although the LIB has been widely applied in some fields and markets, the issues that affect the performance of LIB still have not been completely solved [8]. Particularly, the energy density of LIB still needs to be improved. It remains a challenge to find electrode couples with both high specific capacity and high cycling stability. At the same time, the types and performance of cathode materials are directly related to specific capacity and rate performance of LIB [9,10]. On the other hand, the cycling stability is affected by the degradation mechanism of LIB during operation. The reaction at the electrode–electrolyte interface results in poor cell lifetimes. Therefore, developing the electrode materials of LIB is the primary step.
The cathode is the most expensive and heaviest component in the LIB compared with the anode. Therefore, the cathode currently limits the specific capacity of LIB and mainly determines the cost of LIB [11]. Optimizing the cathode is of great importance for performance improvement of the LIB. At present, the common cathode materials could be classified in three types: (1) olivine structure (such as LiFePO4), (2) spinels structure (such as LiMn2O4) and (3) layered oxide structure (such as LiCoO2, LiNiO2, NCA (LiNiCoAlO2) and NCM (LiNiMnCoO2)) [12]. LiCoO2 with a two-dimensional layered structure has the advantages of simple preparation process, high specific capacity and good cycling performance. LiMn2O4 has a three-dimensional tunnel structure, which is more suitable for the lithiation and de-lithiation reaction, and hence it has decent power capacity and thermal stability. However, it suffers from structure instability, resulting in the easy loss of LIB capacity. By contrast, LiFePO4 exhibits higher practical capacity, better structural stability and good cycle life. Nevertheless, the problems of LiFePO4 include low energy density and poor performance at low temperature. Recently, various metals such as nickel, manganese and aluminum have been combined with cobaltates, including NCA and NCM. However, these types of cathode materials still suffer from similar problems, which limit their further development [13]. To overcome issues of low specific capacity and poor cycling stability in the cathode materials, it has been reported that surface coating is an effective and economical method [14]. Carbon-based materials are a good choice to utilize for coatings, due to their excellent chemical stability and physical properties. Carbon coating aims at offering extra ionic diffusion routes and boosting the transport of electrons through the interface on the cathode surface. Meanwhile, carbon coating could not only control the surface chemical stability of cathode materials and their structure change during lithiation/de-lithiation reaction, but also suppress the adverse reactions between cathode and electrolyte, which caused cycling instability. In addition, carbon coating forms a physical covering layer to diminish the corrosion of electrolytes, which raises the specific capacity, strengthens the thermal stability and prolongs the cycle lifetime of the LIB.
2. Impact of Carbon Coating on Cathode
Surface coating is a well-known optimum approach to protect the cathode material and improve the specific capacity, thermal stability and electrochemical stability of LIB. Carbon-based materials such as porous carbon, graphene, graphene oxide, carbon nanotubes (CNTs) and reduced graphene oxide (rGO) are the most popular coating materials due to the following advantages (Figure 1a,b) [15,16]: (1) superior chemical and electrochemical stability. Due to the reaction of electrolyte and electrode, carbon material has strong electrochemical stability with good resistance to acid electrolyte corrosion, which only shows electrochemical activity at very low potential. Additionally, carbon material, which is not easily oxidated, could protect cathode material from oxygen and moisture in the air. (2) Carbon exhibits unique physical properties such as anisotropic conductivity, low density, high mechanical strength and structural flexibility [17,18]. (3) Excellent electrical conductivity. Carbon with good conductivity is important for coating materials. (4) Low cost. The resource of carbon is sufficient and low-cost, and the fabrication is simple. In addition, the layer thickness and the conductivity of carbon coating could be adjusted by the carbon content and preparation condition [19]. With these excellent properties, carbon is one of the preferred materials for surface coating on the cathode of LIB. The carbon coating has the main following mechanisms (as shown in Figure 1c): (1) Modifying surface chemical stability, (2) Enhancing structural stability and (3) improving Li-ions diffusion. These three carbon-coating modification mechanisms influence and promote each other. In the charge and discharge processes, carbon coatings could relieve the dissolution of transition metals and reduce defects on the surface of cathode materials. It benefits the improvement of interfacial chemical stability, improving the uniform diffusion of Li-ions. Besides, the 3D structure provided by the carbon layer could prevent the structure collapse of the cathode materials, decreasing the particles crack caused by the structure change [20]. This results in the mitigation of metal dissolution again, and the promotion of electron transmission through the interface of the cathode material particles. Meanwhile, the 3D carbon network provides extra electron-conducting routes that facilitate the electron transfer at the cathode surface.
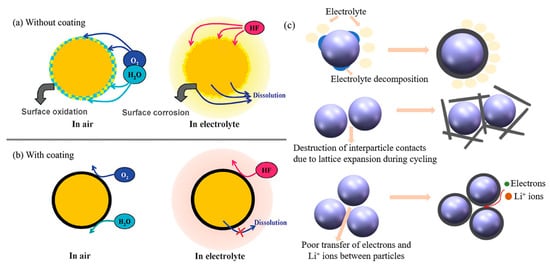
Figure 1. The mechanisms of cathode materials without carbon coating (a) and with carbon coating (b); (c) the modification mechanisms of carbon coating on cathode: modifying surface chemistry, enhancing structural stability and improving Li-ions diffusion.
2.1. Modifying Surface Chemistry
Carbon material with good electrochemical stability serves as a protective barrier to prevent the reaction between cathode material and the electrolyte, and surface oxidation. Besides, through isolating the cathode materials and the electrolyte, residual lithium compounds would be cleared away. On the other hand, the most commonly used lithium salt electrolyte, LiPF6, spontaneously decomposed and produced HF (Hydrofluoric Acid) as follows:
LiPF6→LiF+PF5
PF5+H2O→POF3+2HF
The produced HF by hydrolysis would contribute to the dissolution of transition metals and the corrosion of the surface of active material, resulting in the collapse of the cathode structure. Ultimately, the capacity of LIB declines after long term cycling. Therefore, through carbon coating, the issues of the capacity decline and safety could be relieved effectively.
In addition, the carbon layer separates the active cathode material from the oxygen and moisture in the air. For example, in LiFePO4, such olivine structure materials still suffer from the surface oxidation exposed to the air for long time storage, though it possesses the properties of thermal stability and structural stability [21]. Meanwhile, due to the low electronic conductivity, metal oxides such as Al2O3, CuO and MnO are not suitable for olivine structure material coating. As Pagot et al. demonstrated, the addition of CuO would dilute the cathode active phase, resulting in the weight gain of cathode during the charge/discharge reaction [22]. Although Pagot’s group tried to improve the electronic conductivity of the olivine-structures cathode by doping vanadium, niobium or tantalum high-valence transition metals, the challenge of improving the discharge capacity of the Li-ion battery still needed to be addressed [23]. Therefore, carbon as the chemically stable material could prevent the degradation of the active cathode material in the air, also mitigating the capacity fading in the charge/discharge cycling [17].
2.2. Enhancing Structural Stability
With various unique physical features of anisotropic conductivity, low density, high mechanical strength and structural flexibility, carbon coating easily formed by chemical vapor deposition render as a physical protective layer on the surface of the active cathode material. The undesired surface reaction can be suppressed by the coating layer, which enhances the lifetime of LIB and retains the stability of structure [24]. The structural stability is mainly improved by limiting the cathode volume expansion/shrinkage caused by the phase transformation and providing support for the cathode particles with nanoscale structure.
The phase transition occurred in the process of lithiation and de-lithiation, which creates a new substance with another phase structure. In the procedure of phase transformation, the crystal lattice of the cathode would generate anisotropy expansion, resulting in particles crack. This serious volume expansion will greatly affect the cyclic performances and structural stability of cathode materials. The repeated expansion and contraction of the volume leads to electrode differentiation and the separation of the cathode particles. The hollow carbon material can provide a free space for the volume expansion of the cathode material during the lithium process, ensuring the structural stability of the cathode material and the smoothness of the Li-ions and electron transport channel in the cyclic reaction. Moreover, with good elasticity, carbon could be fabricated as a thin shell on the cathode material surface, which can accommodate the change of volume during Li+ insertion and extraction. Zhang et al. [25] mentioned that the nanoparticles encapsulated elastic hollow carbon spheres can not only avoid the cracking and pulverization of the active material, but also work as buffer and container to enhance the cycle performance and volume capacity.
On the other hand, the nano-crystallization of cathode materials is utilized to shorten the diffusion path of Li-ions and enlarge the specific surface area of the material to provide more diffusion routes for the interfacial reaction. Thus, for the nanoscale active material, carbon coating has positive effect on maintaining the nanomorphology. The preparation of a lot of active materials needs to proceed under high temperature (700–1000 °C) to calcine, meeting the requirement of high crystallization. However, the rapid growth and morphology deterioration occur during the process of calcination. The carbon layer can be a solid barrier between active particles to protect them from becoming large particles during calcination [17].
2.3. Improving Li-Ions Diffusion
Compared with other coating materials, carbon is an excellent electronic conductor. The lithium diffusion of electrode material is one of the most important factors to determine the performance and capacity of LIB, which is extremely essential for cathode material. The mechanisms of Li-ions’ diffusion include diffusion without vacancy, diffusion with vacancy and phase boundary diffusion [26]. Vacancy diffusion plays an important role in most electrode materials, because of the presence of Li vacancies during the charging process. The lattice change of electrode is small in the process of Li insertion and extraction. The phase boundary diffusion including three typical models of the mosaic model, the shrinking core model, and the domino-cascade model refers that Li-ions diffuse from the surface to the particle center by the interface between the lithiation and de-lithiation phases in the limited solubility [27]. To improve the diffusion of Li-ions, coating with an electronic conductive layer for each nanoparticle, the electronic transport length could shrink effectively to the radius of nanoparticles, forming continuous transport channels in the electrode to allow electrons to pass through the surface of each nanoparticle, which effectively reduces the interface resistance between each of the particles [17]. The Li-ions diffusion limited the rate of Li-ions during intercalation/deintercalation process, and the high resistance of the forming material, which could decline the diffusion. Moreover, working temperature also has an important influence on Li-ions diffusion, and the generated heat in the charge/discharge process increases with the increasing square of internal resistance. Decreasing the particle size of cathode material could effectively reduce the internal resistance, however, the transfer of particles along the interfaces would aggravate the resistance. To reduce the interface resistance, carbon plays an important role in filling the grain boundaries, due to its high electrical conductivity and the ability of transferring Li-ions [27]. The electrons transfer has an impact on the Li-ions migration. The carbon coating layer on the active particles could provide fast electrons and are permeable for Li-ions from the electrolyte.
3. Performance of Carbon Coating on Cathode Materials in LIB
Carbon coating sources can be classified into four types: 0-dimensional (0D), 1-dimensional (1D), 2-dimensional (2D) and 3-dimensional (3D) based on the physical morphology structure of carbon. Although the fullerene C60 with a 0D structure is not a common carbon coating source, it remains attractive with its excellent properties of higher electron transport and highly conjugated molecular structure. Liu et al. [37] reported a fluorinated fullerene (C60F48) as a carbon coating source. Cathode materials coated with C60F48 show significantly enhanced LIB performance due to decreased reaction resistance and boosted diffusion of Li-ions. CNTs are one of the most common 1D structure carbon coating sources with excellent mechanical, electrical and chemical properties. Additionally, CNTs enhance the electrical contact between cathode particles and increase stability against the chemical degradation [27]. Graphene, one of the most common coating materials with a 2D structure has a unique electronic feature, which effectively improves the electrical conductivity. Recently, the 3D mesoporous carbon coating materials have gained attraction due to their high electrical conductivity. Such structured carbon coating materials provide a spider-like network for Li-ions and electrons to diffuse faster, improving the rate capacity of batteries.
3.1. Carbon Coating on Olivine Structure Cathode (LiFePO4)
LiFePO4 with the Olivine structure has a theoretical capacity of approximately 170 mAh g−1 and low electrical conductivity of about 10−9 to 10−11 S cm−1 [38]. It exhibited the properties of thermal stability, excellent cycling, low cost, environmental friendliness, and temperature tolerance, making it a promising choice as cathode for powering electric vehicles and consumer electronics. However, Gu et al. [39] illustrated that there are two main factors including the surface amorphization and releasing of oxygen which restrict the further utilization of LiFePO4. With the electrochemical performance tests of LiFePO4 coated with graphite LIB after more than 3300 cycles, Gu’s group found the presence of an amorphous layer with disordered structure destroyed the fast Li diffusion channels. Meanwhile, the structural amorphization caused the electronic structure change of the Fe-ions. Although LiFePO4 has a stable olivine structure with the strong covalent oxygen bonding, the surface of LiFePO4 materials affected the capacity and Li-ions transport of the LiFePO4 electrode. After repeated cycling, the structure of LiFePO4 became amorphous. Meanwhile, the P–O bond strength was weakened, resulting in the release of some oxygen from the surface layer. This is the possible reason for the drop of the Fe valence state in the surface layer. Therefore, the decreasing of capacity and chemical Li-ions diffusion of LiFePO4 was caused by the structural and chemical changes of the surface layer.
Carbon coating is an effective strategy to overcome the shortcomings of LiFePO4 to suppress crystal growth and decline electrode polarization. The electrochemical performance and electrical conductivity of LiFePO4 could be significantly improved by surface modification method with a carbon coating (as summarized in Table 1). It was shown that the common carbon sources used for coating LiFePO4 include CNTs, graphene and monosaccharides. The organic pyrolytic carbon was less used to improve the electrochemical performance of LiFePO4 because of its insufficient electronic conductivity. Luo et al. [40] stated that dispersing CNTs uniformly coating on the surface of LiFePO4 can form a continuous conductive network to reduce electrode polarization and improve cycle capability of cathode, as well as improve the adsorption and immersion of electrolyte to promote the electrode reaction of LIB. Additionally, the carbon coating can act as a buffer layer between active material particles to suppress cracking during reaction. Most notably, the formation of a 3D framework from the 1D CNTs links the active LiFePO4 particles, which can promote the rate capacity performance and cycle stability.
Table 1. Comparison of the cathode performance of LiFePO4 with different coating materials and methods.
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Sucrose | Hydrothermal method and heat treatment | 15.0 | / | 128 mAh g−1 (0.1 C) | No capacity fading (0.1 C, 50 cycles) |
[28] |
| Carbon nanotubes and glucose | Ultra-fine ball milling and spray-drying | 5.0 | / | 127.1 mAh g−1 (10.0 C) | 85.3% (10.0 C, 450 cycles) |
[40] |
| Graphene nanosheet | Chemical vapor deposition | 5.1 | 3.66 | 145 mAh g−1 (0.1 C) | 95.3% (0.1 C,1000 cycles) |
[41] |
| Graphene and sucrose | Solvothermal, drying and calcination | 8.0 | 5 | 163.7 mAh g−1 (0.1 C) 114 mAh g−1 (5.0 C) | 97% (0.1 C, 30 cycles) |
[42] |
| Graphene | Spray-drying and annealing process | 5.0 | 2 | 140 mAh g−1 (0.1 C) | 95% (20.0 C, 1000 cycles) |
[43] |
| Sucrose | Hydrothermal treatment | / | / | 166 mAh g−1 (0.05 C) | 98% (0.1 C, 100 cycles) |
[44] |
| Glucose | Hydrothermal synthesis and annealing process | 1.65 | / | 162 mAh g−1 (0.1 C) | No capacity fading (5.0 C, 50 cycles) |
[45] |
| Graphene oxide and sucrose | Solvothermal method and high temperature solid state reaction | 10.0 | 2–4 | 148.3 mAh g−1 (1.0 C) | No capacity fading (10.0 C, 200 cycles) | [46] |
| New carbon black and polystyrene | Ball-milling and heat treatment | 6.0–8.0 | / | 160 mAh g−1 (0.5 C) | / | [47] |
| Fructose | Hydrothermal process | 8.0 | <5 | Fructose: 98 mAh g−1 (0.1 C) Sucrose: 116 mAh g−1 (0.1 C) Glucose: 63 mAh g−1 (0.1 C) |
/ | [48] |
| Sucrose | ||||||
| Glucose | ||||||
Due to the limited improvement in the poor electrical conductivity of LiFePO4 coated with graphene by a co-precipitation method, Zhou et al. [43] reported a spray-drying and annealing method to prepare LiFePO4 coated with both graphene and glucose-derived amorphous carbon. The LiFePO4 particles were coated with a uniform graphene layer with a 3D network, which enabled fast migration of electrons and Li-ions. This result indicated that LiFePO4 coated with graphene and carbon has a better rate capability and cycle stability. They explained that the glucose-derived amorphous carbon prevented the stacking of graphene sheets, reducing the anisotropy of electronic migration in the graphene layer and accelerating the Li-ions diffusion through the defects in the graphene sheets. Similarly, Jiang et al. [46] demonstrated the addition of rGO could bridge the carbon-coated LiFePO4 nanoparticles (as shown in Figure 2d), improving the homogeneity of the carbon layer and speeding up electrons transfer. The results showed that the rate performance, cycle stability and specific capacity of LiFePO4 with carbon-rGO all were get improvements remarkably. In the mutual effect of carbon and rGO, the discharge capacity of LiFePO4 with carbon-rGO reached 148.3 mAh g−1, which was higher than that of carbon-coated LiFePO4 (Figure 2e). In addition, no capacity fading of LiFePO4 with carbon-rGO was found at the rate of 10 °C in room temperature after 200 cycles (Figure 2f).
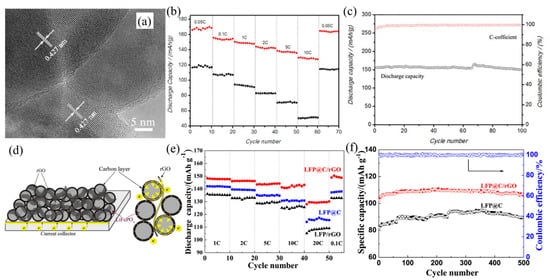
Figure 2. (a) HRTEM images of the hydrothermally synthesized LiFePO4 with P123. (b) Comparison of rate capacity of LiFePO4 with carbon nanostructures and LiFePO4 with carbon microstructures, and (c) cycling performance and Coulombic efficiency of LiFePO4 with carbon nanostructures of the cell with the LiFePO4 with carbon nanostructures as cathode at the rate of 0.1 C. (d) Schematic illustration of 3D conductive network of rGO and carbon layer in LiFePO4 with carbon-rGO composite. (e) The rate performance of LiFePO4 with carbon, LiFePO4-rGO and LiFePO4 with carbon-rGO composites. (f) The cycle life of LiFePO4 with carbon and LiFePO4 with carbon-rGO composite at the rate of 1 C.
Pratheeksha et al. [48] described a simple and low-cost coating method consisting of a one-step hydrothermal process to prepare in situ carbon coated LiFePO4. They also compared the effect on the cathode performance of three different carbon sources, including sucrose, fructose and glucose. The results indicated that this method can form a uniform, thin and highly ordered carbon layer. It was worth noting that using a monosaccharide such as fructose as the carbon source can greatly enhance the electrochemical properties and cycle stability of electrode due to the higher oxygen content and high order of monosaccharide. Controlling the crystal phase of LiFePO4 plays an important role in the electrochemical properties. To study the effect of coating layer on the LiFePO4 crystal orientation and shape, Wang et al. [45] prepared the LiFePO4 coated with glucose as carbon source by a hydrothermal method, exposing the (010) faces in the annealing process. They found that the introduction of carbon content from 1.65 to 6 wt% would change the preferential orientation of the LiFePO4 crystal from the (010) plane to the (100) plane.
Minimizing the size of LiFePO4 particles is an effective approach to promote the electrochemical performance due to the reduced transport distance of Li-ions and electrons, as well as decreasing the phase transition between LiFePO4 particles. Bao et al. [44] reported the plate-like LiFePO4 with carbon coating nanostructures (as shown in Figure 2a) could possess better rate performance compared with the carbon-coated plate-like LiFePO4 microstructures. The discharge capacity of carbon-coated LiFePO4 nanostructure achieved 166 mAh g−1, while that of carbon-coated LiFePO4 microstructure was 117 mAh g−1 (Figure 2b). Moreover, the capacity retention of carbon-coated LiFePO4 nanostructure remained 98% (Figure 2c). Additionally, minimizing the particle size of the cathode materials as well as coating the carbon layer can better boost the rate capacity and cycling stability of cathode materials. Qi et al. [28] illustrated the carbon-coated LiFePO4 microspheres speeded up the charge transfer and improved electrolyte penetration due to the uniform smaller particles and the addition of carbon source. The LiFePO4 with higher carbon content displayed the higher specific discharge capacity of 128.9 mAh g−1 and the better cycling stability than that with low carbon content. Nonetheless, it is still a challenge to get a uniform and high-quality graphene coating layer on the surface of LiFePO4, limiting the enhancement of conductivity. Fei et al. [41] demonstrated that highly crystalline LiFePO4 nanoparticles coated with a homogeneous and continuous graphene nano-shell can be obtained by a solid-state reaction between Fe0 wrapped in a graphene nano-shell and LiH2PO4. It successfully improved the cycle stability and rate capability due to the nanoscale LiFePO4 particles and graphene coating layer, which enables the effective transport and diffusion of Li-ions and electrons.
3.2. Carbon Coating on Spinels Structure Cathode (LiMn2O4)
LiMn2O4 is a typical cathode material with the structure of spinel, which is first commercialized in 1996 [49]. With the theoretical specific capacity of 148 mAh g−1, its discharge voltage reaches 4.15 V. Compared to LiCOO2, LiMn2O4 have a longer cycle life in the range of 1000–1500 cycles, lower cost, and higher rate capacity, but lower energy density in the range of 100–140 Wh kg−1 [49]. Unfortunately, the transformation between spinel structure (cubic symmetry) and halite structure (square symmetry) would be caused by the Jahn-Teller effect. Thereby this distortion of crystal structure makes the LiMn2O4 crystal structure suffers from repeated expansion and contraction. This leads to the deformation and the deterioration of the cyclic performance [50]. The capacity loss can also cause by the instability of the λ-MnO2 phase, the release of oxygen and self-discharge in the de-lithiation process, leading the solvent oxidation [51]. The loss of capacity and Mn-ions of LiMn2O4 cathode material at temperature over 50 °C as well as the transformation of LiMn2O4 phase contribute to the reduced cycle life and existing voltage step [52]. Therefore, the dissolution of Mn in LiMn2O4 is still a major challenge needed to be solved. A coated carbon layer could reduce the dissolution of Mn effectively, and enhance the electrical conductivity of metal oxides.
The cycling performance and stability of LiMn2O4-based batteries are improved by coating with CNTs, graphene-based materials, sucrose, etc. As previously mentioned, the carbon coating technique is a favorable solution to the problems associated with LiMn2O4 including the poor cycling stability, dissolution of Mn2+ into the electrolyte and the reaction between the cathode and electrolyte. LiMn2O4 is a promising cathode material, hence a number of techniques regarding carbon surface modification have been developed (as summarized in Table 2). It is essential that the coating method used does not destroy the structure of LiMn2O4. Jiang et al. [53] proposed a new cyclohexanone hydrothermal method to synthesize LiMn2O4, which can control the particle size in the synthesis process. The results indicated that the CNTs coating layer can be optimized to achieve excellent electrochemical performance without damaging the crystal structure of LiMn2O4 cathode material. Besides, in order not to destroy the crystal structure of LiMn2O4, Li et al. [54] also indicated that the host structure was not damaged after coating the cathode material with rGO by the precipitation method (as shown in Figure 3a). They proposed that a binding site could be provided by the oxygen-containing group of graphene oxide for the precursor metal ions, which could reduce the agglomeration of LiMn2O4 and shorten the diffusion path length of Li-ions, and in favor of the transport of Li-ions. Compared to the LiMn2O4 without coating rGO, the initial discharge capacity of LiMn2O4/rGO increased by around 35 mAh g−1 (as shown in Figure 3b), and the capacity retention rate was obviously boosted (as shown in Figure 3c).
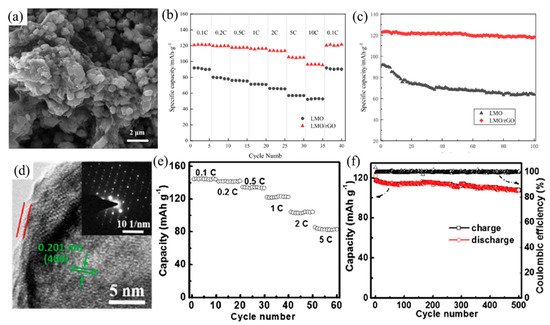
Figure 3. (a) The SEM images of LiMn2O4/rGO; (b) discharge rate capability at different current densities; (c) cycling performance curve of LiMn2O4 and LiMn2O4/rGO at 0.2 C. (d) The HRTEM diagram of LiMn2O4 with 10 wt% carbon; (e) rate capability under variable current rate; (f) cycling performances of LiMn2O4 with 10 wt% carbon at 1 C.
Table 2. Comparison of the cathode performance of LiMn2O4 with different coating materials and methods.
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Glucose | High temperature solid-state method | 10.0 | 3 | 132 mAh g−1 (0.1 C) |
90% (1.0 C, 500 cycles) |
[31] |
| Glucose | Hydrothermal method and heat treatment | 10.0 | 1.5 | 138.5 mAh g−1 (0.1 C) |
97.76% (0.1 C, 100 cycles) |
[53] |
| Reduced graphene oxide | Ball-milling and calcination | 5.0 | / | 127 mAh g−1 (0.1 C) |
96.2% (0.2 C, 100 cycles) |
[54] |
| Carbon nanotubes | High temperature solid-state reaction | 5.0 | / | 110.3 mAh g−1 (1.0 C) |
98% (1.0 C, 20 cycles) |
[55] |
| Liquid-polyacrylonitrile (LPAN) graphene-like membrane | Solid-state ball-milling | 20.0 | 3 | 131.1 mAh g−1 (0.1 C) |
96% (0.1 C, 50 cycles) |
[56] |
| Carbon black | Wet slurry and heat treatment | 4.0 | / | 107 mAh g−1 (0.5 C) |
92.3% (0.5 C, 36 cycles) |
[57] |
| Graphene oxide flakes | Wet chemical and heat treatment | 5.0 | / | 98 mAh g−1 (20 mAh g−1 current density) |
91.2% (20 mAh g−1 current density, 100 cycles) |
[58] |
| Polydopamine | Polymerization process of dopamine and heat treatment | 0.25 0.65 | / | 113.3 mAh g−1 (70 mAh g−1 current density) 93 mAh g−1 (70 mAh g−1 current density) |
51.7% (140 mAh g−1 current density, 36 cycles) 73.2% (140 mAh g−1 current density, 36 cycles) |
[59] |
| Ethanol | Hydrothermal process and annealing treatment | 0.27 | / | 129.4 mAh g−1 (0.5 C) |
90% (30.0 C, 1500 cycles) |
[60] |
| Poly (N-vinylformamide) |
Mixing in solvent and heat treatment | 5.0 | 2–3 | 121 mAh g−1 (1.0 C) |
74% (5.0 C, 1700 cycles) |
[61] |
Zhang et al. [55] proved that LiMn2O4 coated with CNTs can exhibit better electrochemical performance than LiMn2O4 without coating because the CNTs coating layer can decrease the contact area between the cathode material and electrolyte. This mitigates the dissolution of manganese, improving the electrochemical cycle performance. Although the discharge capacity of LiMn2O4 coated with CNTs was lower than that of bare LiMn2O4 at a low cycle rate, it had better electrochemical performance at a high cycle rate. However, due to the high cost of graphene and CNTs, there is an increasing research focus on carbon coating materials with simple preparation and low production cost. Zhuo et al. [56] reported a graphene-like membrane prepared using liquid polyacrylonitrile as a substitute for graphene. The results showed that the graphene-like membrane with a layered carbon structure will not affect the crystal structure of LiMn2O4 particles, which improves the discharge capacity and cycling stability. Additionally, dopamine as the carbon source had been reported, and the result illustrated that the carbon coating layer could be fabricated uniformly due to the easy and homogeneous polymerization process from the dopamine solution. However, the oxygen loss in the carbon-coating process led to the deterioration of the phase integrity of LiMn2O4. Furthermore, LiMn2O4 with a nanostructure such as nanosheets, nanoparticles, porous structure and nanowires have been investigated. In particular, the 1D nanomaterial has a large surface area, good electron conduction and anisotropic Li-ions diffusion paths, which can shorten the diffusion distance between electrons and Li-ions. Cao et al. [31] illustrated a new approach to preparing carbon-coated single-crystalline LiMn2O4 nanowire, via agglomeration into the bulk of nanoparticles at high temperature (shown in Figure 3d). This 1D form of material can make Li-ions diffusion paths possess anisotropy, which showed the increasing discharge capacity of 132 mAh g−1 at 0.1 C (Figure 3e) and a retention of 90% (Figure 3f) of the initial capacity at 1 C after 500 cycles. Carbon layer can not only inhibit the dissolution of cations to enlarge the capacity of battery, but also sustain the final LiMn2O4 nanowires morphology during the lithiation/de-lithiation reaction to improve the long-term cycling stability.
3.3. Carbon Coating on Layered Oxide Structure Cathode
3.3.1. Carbon Coating on LiCoO2 Cathode
LiCoO2 is a cathode material with a typical layered oxide structure, with a high theoretical capacity of up to about 274 mAh g−1. However, the charging voltage of LiCoO2-based LIB was limited below 4.25 V and the practical capacity of LiCoO2 is only 140 mAh g−1, which only accounts for 50% of the total capacity [62]. In the high voltage operation, the failure mechanism of LiCoO2 could be classified into three aspects: bulk phase transition, surface degradation and inhomogeneous reaction [63]. The presence of the irreversible phase transition and particle cracks caused by the change of structure and volume in the Li-ions deintercalation/intercalation leads to capacity loss. The previous study indicated that the structure of LiCoO2 would change from O3 hexagonal phase to H1-3 phase when the cut-off voltage is above 4.45 V [64], resulting in the cracks. Besides, surface degradation can be caused by the impedance growth of LiCoO2 electrodes including the continuous formation of the cathode electrolyte interphase, irreversible surface phase transitions, O2 loss, and Co dissolution [63]. Based on the inhomogeneous reaction mechanism, the differences of Li diffusion dynamics make the state of charge of different particles or different part of a particle inhomogeneous. Hence, such inhomogeneous distribution of the state of charge caused serious deformation and stress, resulting in the splintering of electrode and particles and the loss of capacity. Furthermore, due to the instability of the LiCoO2 surface under the condition of high pressure, it is easy to generate an unstable cathode electrolyte interphase, which is decomposed during cycling process, contributing to the poor cycling performance [63]. Although LIB with LiCoO2 has the better stable capacity, it was reported that LiCoO2 cathode had safety issues that low thermal stability leading to a runaway reaction and burst into flames [65].
As discussed before, the degradation of LiCoO2 is caused by bulk phase transition, surface degradation and inhomogeneous reaction. A lot of studies about LiCoO2 coated with carbon from different carbon sources and using different approaches have been reported (as shown in Table 3).
Table 3. Comparison of the cathode performance of LiCoO2 with different coating materials and methods.
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Carbon black | Sol-gel method | 1.0 | / | 145 mAh g−1 (1.0 C) | / | [66] |
| Sucrose | Milling and calcination | 5.0 | / | 130 mAh g−1 (0.1 C) | / | [67] |
| Plated-shape graphite | Ball-milling and drying | 20.0 | / | 80 mAh g−1 (0.1 C) | / | [68] |
| Graphite | Milling and drying | 10.0 | / | 220 mAh g−1 (0.1 C) | / | [69] |
| Graphene nanosheet | Dispersing in solution and evaporation | 2.1 | / | 180.8 mAh g−1 (0.1 C) | 88.5% (0.1 C, 100 cycles) |
[70] |
| Graphene quantum dots | Liquid phase method and filtrating and drying | 1.0 | 10 | 182.7 mAh g−1 (0.1 C) | 82.8% (0.5 C, 100 cycles) |
[71] |
| MOF-derived carbon | High temperature solid-state method | 14.03 | 5 | 193.4 mAh g−1 (0.1 C) | 89.1% (0.1 C, 200 cycles) |
[72] |
| Carbon black | Mixing solvent and drying | 6.0 | 10 | 170–177 mAh g−1 (0.1 C) |
60.3% (0.1 C, 100 cycles) |
[73] |
| Super-aligned Carbon nanotubes | Ultrasonication and co-deposition technique | 5.0 | 20 | 151.4 mAh g−1 (0.1 C) |
98.4% (0.1 C, 50 cycles) |
[74] |
| Carbon black | Pyrolysis of resorcinol | 0.88 | 2 | 147 mAh g−1 (0.3 C) | / | [75] |
Actually, carbon coating was rarely reported to modify LiCoO2 before, because LiCoO2 is easily reduced by carbon to CoO or Co3O4 in the high temperature calcination [67]. Thus, it was reported earlier to coat LiCoO2 with carbon black to reduce the amount of conductive agent. However, a carbon black coating layer mainly worked as physical protection without strong contact with LiCoO2 [67]. Except for the thermal decomposition method, various effective approaches have been introduced, including chemical vapor deposition and mechanical milling. It was suggested by Kwon et al. [68] that the post-treatment technique was better for coating LiCoO2 with carbon, and the mechanical milling process possesses the advantages of simple operation, relatively low production cost and providing close contact between LiCoO2 and carbon. By contrast, the method of solution mixing is not suitable for the preparation of carbon coatings which would cause an inhomogeneous carbon layer due to the large density difference between LiCoO2 and carbon. By testing the LiCoO2 coated with platelet-shaped graphite, they demonstrated that the increased specific surface area caused by the reduction of the particle size could provide a larger contact area for the electrode and electrolyte. Additionally, the uniform and thin graphite coating layer allowed electrons and Li-ions to pass through the composite, improving the capacity and cyclic voltammograms. Subsequently, Kwon et al. [69] also indicated that the milling time had an important impact on the performance of the carbon coating. Short-time ball-milling can provide a uniform and inmate carbon layer, improving the electrochemical performance by shortening the diffusion length of Li-ions. In comparison, long-time ball-milling increased the number of defects in the structure of carbon, resulting in a thick carbon layer and decreasing the electrochemical performance.
Wang et al. [70] showed that graphene nanosheets of small size can coat the surface of LiCoO2 with a homogenous distribution and without much aggregation, accelerating the transport of electrons and diffusion of Li-ions due to the high electronic conductivity of the graphene network. Nanoscale graphene particles have a high specific surface area and excellent electronic and ionic conductivities. Sun et al. [71] reported that LiCoO2 coated with graphene quantum dots by a simple liquid phase approach showed better cycle stability without the presence of micro-cracks. Luo et al. [74] developed LiCoO2 coated with highly aligned CNTs to create a three-dimensional conductive network through an ultrasonication and co-deposition method. The material exhibited good electronic conductivity, high flexibility, high energy density and excellent cycle stability. Liang et al. [73] suggested that atomic layer deposition was an alternative technique to coat carbon on the surface of LiCoO2 because atom layer deposition enables accurate control over the thickness of the carbon layer, and it forms a uniform, conformal and thin carbon film (Figure 4a,b). Figure 4a,b showed the schematic diagram of LiCoO2 particles and electrode coated by conductive carbon with ALD coating method, respectively. They found that the capacity rate of LiCoO2 electrode coated with carbon was remarkably improved at low current density (<0.6 C), while the LiCoO2 particles coating achieved the higher capacity at the high current density (Figure 4c). Besides, the most important benefit is that the process uses a relatively low temperature, hence this method would not damage the structure of the carbon and electrode during the preparation. Recently, Lin et al. [72] provided a new strategy of in situ constructing MOF-derived to prepare LiCoO2 with carbon-coated core-shell structure by elevated-temperature solid-state annealing. Through several annealing temperature comparison, they found that carbon-coated LiCoO2 formed at the annealing temperature of 700 °C (Figure 4d) could exhibit superior electrochemical performance. Figure 4e,f showed the carbon-coated LiCoO2 synthesized by the 700 °C annealing temperature has the capacity retention of 89.1% at the current density of 1 C after 200 cycles and the discharge capacity of 193.4 mAh g−1 at the current rate of 0.1 C.
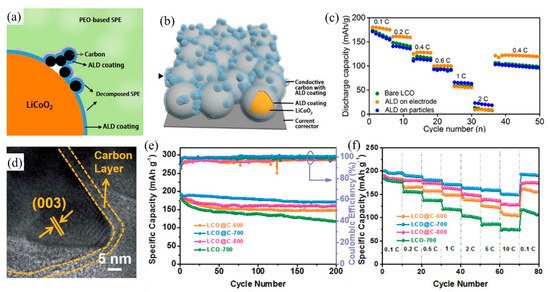
Figure 4. (a) The working mechanism of the protected LiCoO2 with carbon coating in the ASSLBs after extensive charge/discharge cycles; (b) Schematic diagram of the LiCoO2 electrode where both LiCoO2 and conductive carbon are protected. (c) Rate performance of the ASSLBs. (d) HRTEM image of the carbon layer of LiCoO2 at a scan rate of 0.1 mV s1. (e) Cyclability at a current density of 1 C, and (f) rate capability at a current density ranging from 0.1 C to 10 C of LiCoO2 with carbon coating
3.3.2. Carbon Coating on LiNiO2 Cathode
LiNiO2 is considered as the alternative to LiCoO2 owing to the properties of low cost and a high theoretical capacity of 274 mAh g−1 at a reasonable voltage range between 2.6 and 4.2 V [76]. However, LiNiO2 could not be commercialized due to its complicated synthesis process, of which the composite of material is difficult to control precisely [77]. In addition, Ni2+ ions tend to hinder the diffusion of Li-ions, leading to instability of LiNiO2. Firstly, the stoichiometric LiNiO2 is difficult to prepare by solid-state reaction method under high temperature due to the high vapor pressure of lithium during the process of calcination, leading to the loss of Li from the host structure, and hence the formation of a non-stoichiometric structure. Such a non-stoichiometric structure makes LiNiO2-based LIB have the low initial capacity and the serious problem of capacity loss [78]. On the other hand, when charging up to high voltage, the structure of LiNiO2 will be changed due to the formation of NiO2 phase caused by the irreversible phase transitions. The inactive NiO2 phase also reduces the capacity.
With a similar structure, LiNiO2 has a higher reversible capacity than LiCoO2. Due to the difficulty of oxidation of Ni2+ to Ni3+ during the high temperature synthesis process, it is challenging for large scale preparation. Vandenberg et al. [79] prepared in situ carbon-coated LiNiO2 by a microwave-assisted synthesis method, exhibiting an initial specific charge of around 270.5 Ah kg−1 at 1 C with an about 98% charge retention at 1 C after 1500 cycles (as shown in Figure 5). However, few studies on carbon-coated LiNiO2 have been reported, because coating metallic oxide materials would be more common. Meanwhile, in order to improve the cycling performance and thermal stability of LiNiO2, cobalt doping attracts increasing research attention such as LiNi0.8Co0.2O2 [80].
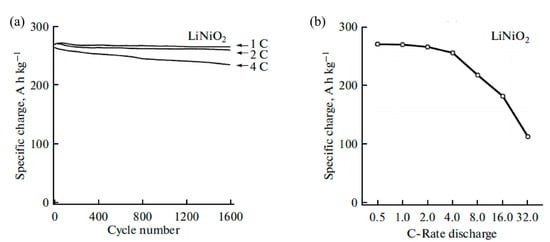
Figure 5. (a) Electrochemical specific charge of LiNiO2; and (b) dependence of the specific charge of LiNiO2.
3.3.3. Carbon Coating on NCM (LiNixCoyMn1−x−yO2) Cathode
To solve the problem of LiNiO2, the strategy of using cobalt, manganese or aluminum as the substitution to decrease the fraction of nickel has been developed, thereby the new composites LiNixCoyMn1−x−yO2 (NCM) were developed by the Ohzuku et al. in 2001 [81]. With the advantages of relatively low cost, better thermal stability and high capacity, the theoretical capacity of LiNi0.8Co0.1Mn0.1O2 (NCM 811) could reach to about 200 mAh g−1 [82]. However, NCM exited a major problem of capacity loss due to the degradation of NCM material from nano-scale to micro-scale in the process of discharge and charge [83]. In addition, the oxygen released from NCM with high degree of lithium deintercalation reacts with the organic electrolyte, resulting in the poor thermal stability. In addition, the increasing solid-electrolyte interfacial impedance due to the reduction of Ni4+ led to poor cycle life [82]. It has been suggested that surface modification is an effective method to improve the performance of NCM. Surface coating can be classified into two types including coating on primary particle level and coating on secondary particle level. It is an effective strategy to prevent the reaction between NCM cathode and electrolyte, also restrains the oxygen evolution of NCM during cycles [81].
To prevent the reaction between NCM cathode and the organic electrolyte, a carbon coating as a physical protection layer and chemical barrier can effectively improve thermal stability of NCM and enhance the electrochemical performance by increasing the Li-ions transport and electronic conductivities. Recently, a lot of different coating methods including chemical vapor deposition, atomic layer deposition and physical vapor deposition, and different coating materials such as graphene, CNTs and sucrose have been investigated, as summarized in Table 4. Chen et al. [84] discussed the issues of carbon coating techniques on Ni-rich cathodes, including that the coating process needed to be conducted without water, and the oxidation of carbon in oxidative environments above 500 °C since the synthesis of NCM generally proceed under an oxygen atmosphere at high temperatures, leading to the oxidization of organic compounds to CO. Therefore, water-soluble carbon sources such as sucrose and glucose are not suitable for coating Ni-rich cathodes at high temperatures and it is still challenging to form a continuous and uniform carbon coating.
Table 4. Comparison of the cathode performance of NCM with different coating materials and methods.
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Polymers | Chemical wetting method and heat treatment | 0.39 | 4 | 191 mAh g−1 (0.5 C) | 98.74% (0.2 C, 100 cycles) |
[84] |
| Carbon nanotubes and graphene | Wet chemical method | 10.0 | / | 187 mAh g−1 (0.5 C) | 93.8% (1.0 C, 50 cycles) |
[85] |
| Sucrose | Chemical vapor deposition | 2.5 | 6 | 218.2 mAh g−1 (0.1 C) | 94.78% (0.1 C, 100 cycles) |
[86] |
| Carbon nanotubes | Wet chemical method | 0.01 | 4 | 202.6 mAh g−1 (0.5 C) | 84.8% (0.5 C, 500 cycles) |
[87] |
| Active carbon | Sol-gel route | 4.1 | 10 | 191.2 mAh g−1 (0.5 C) | 90.3% (1.0 C, 100 cycles) |
[88] |
| Super-P carbon black | RAM (resonant acoustic mixer) and heat treatment | 0.5 | 0.89–1.23 | 188.6 mAh g−1 (0.5 C) | 87.8% (0.5 C, 80 cycles) |
[89] |
| Single-walled carbon nanotubes | Chemical wetting method and heat treatment | 5.0 | 8 | 160 mAh g−1 (0.5 C) 130 mAh g−1 (5.0 C) | 92% (5.0 C, 500 cycles) |
[90] |
| Carbon black | Electrostatic spraying | 1.0 | / | 156 mAh g−1 (0.2 C) | 80% (0.2 C, 300 cycles) |
[91] |
| Graphene ball | Chemical vapor deposition and wet slurry method | 1.0 | 5 | 191.6 mAh g−1 (0.1 C) | 97.3% (1.0 C, 100 cycles) |
[92] |
| Soybean oil | Solid-state method | / | 5 | 159 mAh g−1 | 95% (100 cycles) |
[93] |
For the coating method of NCM cathode, chemical vapor deposition (CVD) and inverse microemulsion routes have been reported. Hou et al. [86] illustrated that the chemical vapor deposition technique could fabricate a more uniform and high-quality carbon coating layer compared with the traditional coating method of heat evaporation, thus CVD is considered a simple approach to achieving uniform carbon coating. However, the complex process and expensive equipment of these coating methods make them difficult to use in large-scale production. Yang et al. [88] reported a simple method of using highly absorbing activated carbon to absorb the Li-ions and form a uniform carbon layer on the surface of NCM particles. In addition, Al-Shroofy et al. [91] developed a lower cost, high throughput method of solvent-free dry powder coating, compared to the conventional wet slurry-based electrode manufacturing method. Their group made a comparison of the effect of carbon coating by two fabrication methods including dry powder coating and wet-slurry coating, which showed the carbon coating made by dry powder had a higher specific capacity. Moreover, this method also mitigated the fabrication problems of high cost and environmental issues, because dry powder coating can reduce the production cost by simplifying the preparation steps and saving drying time, and dry powder coating could relieve the pollution from solvent.
For the coating materials of NCM cathode, graphene, CNTs and carbon black are common carbon coating materials. Li et al. [85] reported that unlike coatings of either graphene or CNTs alone, coating both CNTs and graphene enables the formation of a 3D conductive spider web framework in the cathode. CNTs provide an effective link between graphene and NCM to enable fast transportation of electrons and Li-ions, and lower electrode polarization, improving rate capacity and increasing cycling stability. Nguyen et al. [93] illustrated that using soybean oil as the carbon coating source (Figure 6a,b showed the TEM image and FE-SEM images of NCM/C, respectively), exhibited the outstanding advantages of simple fabrication and low cost. As well as, it easily formed a coating layer through an emulsion reaction, and improved the specific capacity and cycling stability. At the same time, they discovered the capacity of NCM/C battery reached to 159 mAh g−1 at the 2nd cycle with the gradual wetting of the electrolyte, and maintained 95% of the capacity at 0.1 C after 100 cycles (Figure 6c). Furthermore, Yang et al. [87] demonstrated that a Li3PO4 (LPO) and CNTs multi-functional coating on the surface of NCM showed high electronic and ionic conductivities, high-rate performance and excellent cycle stability (Figure 6d). Among the pristine NCM, LPO-NCM and CNT-LPO-NCM, the CNT-LPO-NCM showed the most outstanding initial discharge capacity of 202.6 mAh g−1 and the best cycling stability with capacity retention of 84.8% at 0.5 C after 500 cycles (Figure 6e). NCM, Li3PO4, CNTs and the electrolyte form a four-phase cathode electrolyte interface to provide rich electronic pathways and ions channels to improve the electronic conductivity and prevent the corrosion of HF. The thickness of carbon coating layer made a difference in the capacity and cycle performance of NCM. Sim et al. [89] reported the initial discharge capacity of carbon black coated NCM (188.6 mAh g−1) was lower than that of the pristine NCM (192.8 mAh g−1). The carbon coating layer works as an obstacle because of higher electrode polarization resulting in lower specific capacities while carbon-coated NCM had higher capacity retention and better cyclability.
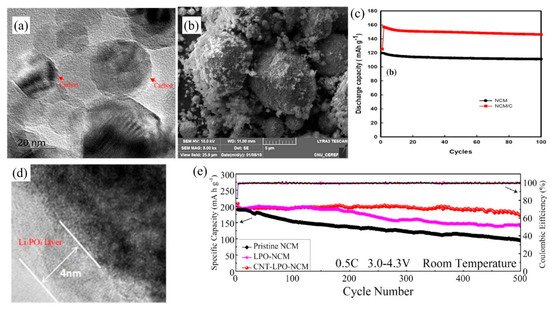
Figure 6. (a) TEM images of the NCM coated with carbon layer; (b) FE-SEM images of the NCM/C powder; and (c) discharge capacity of the NCM/C-based LIB. (d,e) TEM images of the CNT-LPO-NCM; and cycling performances of pristine NCM, LPO-NCM and CNT-LPO-NCM at 0.5 C separately in the voltage range of 3.0–4.3 V.
3.3.4. Carbon Coating on NCA (LiNixCoyAl1-x-yO2) Cathode
NCA (LiNixCoyAl1-x-yO2) is a promising alternative for next-generation LIB with high energy density. The advantages of high reversible specific capacity, high cyclic stability, low cost and structure stability because dopant (aluminum) make it successfully applied in electric vehicles including Tesla Mode 3 [94]. LiNi0.80Co0.15Al0.05O2 (NCA8115), one of the most common NCA cathode material, could achieve a specific capacity of 265 mAh g−1 with high specific energy of about 200 mAh g−1, and it was reported the practical capacity could reach about 199 mAh g−1, which was higher than that of LiCoO2 at an average voltage of 3.7 V [95]. However, it exists the problems of low thermal stability, strict manufacturing condition and residual lithium compounds during synthesis [96]. For the performance deterioration of NCA, there are main four causes including cationic mixing, phase transition, residual lithium compounds, and microcracks, resulting in the presence of deficient cycling performance and thermal stability. Firstly, due to the similar radius between Li+ and Ni+, it is easy for Ni+ to migrate to Li+ layer and occupy Li+, which makes the thermal instability of the structure of NCA. Moreover, due to the instability of Ni3+ and Ni4+ at high temperature, the reaction between HF released by the electrolyte and these high oxidation state Ni-ions easily occurs, leading to the structural change and material degradation which affect the capacity and cycle performance of NCA material [97]. Besides, cations mixing makes an impact on the layered-spinel-rock salt phase transition, contributing to the loss of oxygen from the cathodes and the formation of a thick and highly resistive layer improves the transfer impedance. Meanwhile, the capacity of NCA-based LIB decreased with increasing extent of phase transition. Moreover, the fabrication of NCA and the reaction of LiNiO2 with H2O and CO2 from the air both produce the residual lithium compounds on the surface of NCA cathode, leading to the slurry gelation and battery swelling. Furthermore, the microcracks caused by the anisotropic volume changes form the excessive solid electrolyte interface to prevent the diffusion of Li-ions [98]. Therefore, surface coating providing physical protection is an effective strategy to modify the performance of NCA cathodes, preventing the oxygen evolution and reactions between electrode and electrolyte.
The main problems of NCA include cationic mixing, phase transition and microcracks. Carbon coating is a proven strategy to solve these problems by preventing corrosion by HF and suppressing oxygen volatilization, hence improving the conductivity, enhancing electron transport, reducing polarization and improving the stability of the surface (summarized in Table 5).
Table 5. Comparison of the cathode performance of NCA with different coating materials and methods.
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Diamond-like carbon | Chemical vapor deposition method | 5.0 | 4.3 | 120.7 mAh g−1 (0.05 C) | 90% (0.1 C, 100 cycles) |
[99] |
| Multi-walled carbon | high-powder ultrasonic stirring | 0.5 | / | 205.6 mAh g−1 (0.1 C) | 91.7% (2.0 C, 800 cycles) |
[100] |
| Aniline and phytic | chemical wetting and heat treatment | 1.0 | 8 | 190 mAh g−1 (1.0 C) | 90.7% (1.0 C, 200 cycles) |
[101] |
| Reduced graphene oxide | Mechanical wet ball-milling method | 1.0 | 3.9 | 196 mAh g−1 (0.2 C) | 91.7% (1.0 C, 100 cycles) |
[102] |
| Sucrose | Chemical wet and heat treatment | 1.0 | 4 | 250 mAh g−1 (0.1 C) | 88.3% (1.0 C, 200 cycles) |
[103] |
| Glucose | 1.0 | 3 | 225 mAh g−1 (0.1 C) | 70.4% (1.0 C, 200 cycles) |
||
| Graphene | Wet slurry and heat treatment | 4.5 | <20 | 190 mAh g−1 (0.1 C) | 60.5% (1.0 C, 200 cycles) |
[104] |
| Graphene | Pickering emulsion process | 0.5 | <10 | 191 mAh g−1 (0.1 C) | 70% (1.0 C, 250 cycles) |
[105] |
| Graphite sheets | Mixing and cladding process by a mechanical fusing machine | 8 | / | 181 mAh g−1 (0.2 C) | 85% (0.5 C, 400 cycles) |
[106] |
| Polyacryloni-trile (PAN) | Chemical wet and high temperature heat treatment | 4 | 5 | 180.2 mAh g−1 (1.0 C) | 98.4% (1.0 C, 100 cycles) |
[107] |
| Graphene | Sonication and “collage” technique | 1.0 | 3.1 | 208 mAh g−1 (0.1 C) | 72% (0.5 C,100 cycles) |
[108] |
Various coating methods have been demonstrated, and the carbon cladding can be classified into three types: core-shell structure cladding, ultrathin film cladding and rough cladding [106]. The structure of carbon core-shell can indeed improve the capacity and stability of the cathode material; however, the cladding process is complicated and includes two-step co-deposition. In addition, atomic layer deposition is one of the most common methods to prepare a carbon coating layer, which can form a homogeneous and continuous thin carbon film. However, this technique is limited by the target source. Therefore, Zhao et al. [106] proposed a coating method of mixing and rough cladding using a mechanical fusing machine. Although the rough cladding technique is simple and easy to operate, this method still cannot fabricate a continuous carbon layer, unlike the other two cladding methods. On the other hand, Yu et al. [100] illustrated that CNTs coated on the surface of NCA by the traditional method of thermal treatment can effectively shorten the channel length for Li-ions transport to improve the electronic conductivity. Carbon thermal reduction would result in damage to the structure of the cathode material during the high temperature heat treatment. Therefore, there is still a need to develop a coating method to form a uniform and continuous carbon layer which can ensure the structural integrity of the cathode material and meet the requirements for mass production. To this end, Park et al. [105] proposed a new carbon coating technique based on the Pickering emulsion processing, which has high scalability, throughput and amenability to recycling. It can greatly decrease the amount of carbon conductive additive and polymer binder, and hence increase the active material percentage and packing density. Furthermore, Park et al. [108] developed a new coating technique, the “collage” technique, which can obtain a uniform carbon coating layer whilst avoiding high-temperature processing and damage to the cathode material. The results showed that this technique can not only provide a sufficiently conductive network, but also increase the electrode density. These new techniques can provide a useful idea for further development of the carbon coating method.
For the coating materials of NCA cathode, Visbal et al. [99] proposed a new protective coating layer, diamond-like carbon, to decrease the interface resistance between the electrode and sulfide solid electrolyte. They proved that the diamond-like carbon coating works both as a protection layer and Li-ions mediator. However, they also illustrated the specific capacity of the cathode was affected by the coating layer thickness, because Li-ions need to pass through the diamond-like carbon layer. Gao et al. [101] also indicated that the discharge-specific capacity and initial efficiency decreased with increasing thickness of the coating layer, which is caused by the reduction of Ni3+ to Ni2+, leading to a higher degree of lithium–nickel intercalation in the cathode. In addition, Liu et al. [103] found both the discharge capacity and the capacity retention of NCA coated with sucrose as carbon source (Figure 7a) were more outstanding than that of NCA coated with the same content of glucose as carbon source. As Figure 7b shown, the initial discharge specific capacity of sucrose-coated NCA reached over 250 mAh g−1 at current density of 0.1 C, as well as the capacity retention of sucrose-coated NCA was 88.3% after 200 cycles at 1 C (Figure 7c). The authors indicated these results attributed to the relatively loose sucrose coating layer with the larger size pores, making the carbon layer has a large specific surface area. It contributed to the movement of Li-ions during the lithiation/de-lithiation process and mitigated the erosion of the electrolyte. To solve the issue of phase transition of NCA, it was reported that an rGO coating layer could suppress the rock salt NiO phase of the NCA nanoparticles and enhance electron transfer and Li-ions diffusion. This fabrication strategy of mechanical wet ball-milling made conformal coating of rGO on the surface of NCA nanoparticles [101]. Generally, it is difficult to form a continuous carbon layer with CNTs and graphene as the carbon source by the conventional methods, and the high cost of these materials makes them only suitable for laboratory research.
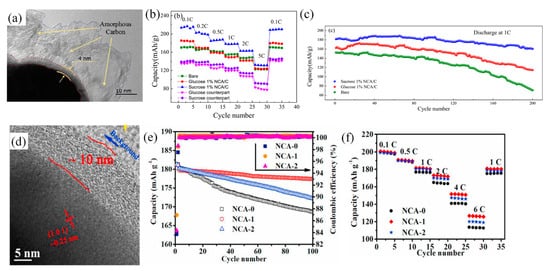
Figure 7. (a) TEM images of 1% sucrose-coated NCA; (b) rate test of coated and uncoated NCA; and (c) cycle performance test of coated and uncoated NCA at 1 C. (d) TEM images of the NCA coated with 8.0 wt% content of the PAN solution. (e) Charge/discharge cycle performances of the NCA cathodes; and (f) rate cycles of the NCA cathodes.
On the other hand, in the high temperature process, glucose and sucrose are not suitable as the carbon coating sources due to the generation of H2O and CO2 from carbon, which would damage the structure of the cathode. Thus, Feng et al. [107] proposed using polyacrylonitrile (PAN) as the carbon coating source, which successfully formed a continuous carbon coating layer on the surface of NCA (Figure 7d). Although, NCA-2 (8.0 wt% content of PAN solution) possessed the highest initial discharge capacity of 181.2 mAh g−1, while the NCA-1 (4.0 wt% content of PAN solution) exhibited the highest capacity retention of 98.4% under 1 C current density after 100 cycles (Figure 7e) and the highest capacities at different rates (Figure 7f). The results showed a significant improvement of the electrochemical performance, including the restraint between NCA electrode and environment moisture conditions and the promotion of interfacial dynamics of cathode/electrolyte. The further relationship between carbon content and final electrochemical performance was confirmed.
For further research, it is essential to take the coating material and coating method into consideration. For NCA and NCM, which need to be prepared by heat treatment, a polymer as the carbon source is a good choice. Additionally, a growing number of coating methods aimed at simplifying the process and preparing a continuous and uniform carbon coating layer have been reported.
4. Limitation of Carbon Coating Method
Although carbon coating on the surface of LIB cathode can effectively enhance the electrochemical performance of battery, several limitations of carbon coating still need to be solved. Actually, many researchers focused on the effect of carbon coating materials and coating methods on the cathode performance, the more comprehensive studies on the influence of different factors such as the suitable coating layer, homogeneity, and the diffusion behavior of electrons are still limited. Therefore, the following aspects should be improved in the further study. (1) The uniformity and integrity of carbon coating layer. It is important to make sure the carbon coating layer completely covers the cathode material particles, and ensures the integrity of carbon coating without structural destruction with increasing cycle times. (2) The surface characteristics of cathode material including pH values and different charged situation of the particle surface. The surface of the cathode material and the coating material should have good compatibility to well modify the cathode material. As Yang et al. mentioned, the addition of p-phenylenediamine (PPD) can remain the reaction pH value to form impurity-free LiMnPO4, which shortens the Li-ion diffusion length. In addition, LiMnPO4 coating with nitrogen-doped carbon by pyrolysis of the poly (p-phenyleneterephthalamide) (PPTA) and poly (p-phenylene decanamide) (PPDA) showed better conductivity, contact ability and surface capacitance than that of coating with sucrose [109]. (3) Surface modification technologies, such as the combination of coating and doping. Thus far, such studies on combining the surface coating methods have been reported. A recent study involved the strategy of LiNi0.6Co0.2Mn0.2O2 (NCM622) with nitrogen-doped carbon coating [110]. It proved that the nitrogen-doped carbon coated NCM622 displayed an improvement of rate performance and capacity retention (92%). The carbon layer with nitrogen doping contributes to achieving the higher degree of cation ordering, moderating the reactions between the cathode and electrolyte as well as increasing electronic conductivity. (4) The selection of carbon content in coating. It is hard to determine the suitable carbon content in coating. For example, the high carbon coating content for LiFePO4 will cause the decline of Li+ diffusion coefficient, and reduction of specific capacity. By contrast, if the carbon content is insufficient, the positive effect of coating layer on LiFePO4 will be indistinctive.
This entry is adapted from the peer-reviewed paper 10.3390/nano12111936
References
- Zhang, Q.; Liang, Q.; Liao, Q.; Yi, F.; Zheng, X.; Ma, M.; Gao, F.; Zhang, Y. Service behavior of multifunctional triboelectric nanogenerators. Adv. Mater. 2017, 29, 1606703.
- Liang, Q.; Yan, X.; Liao, X.; Cao, S.; Zheng, X.; Si, H.; Lu, S.; Zhang, Y. Multi-unit hydroelectric generator based on contact electrification and its service behavior. Nano Energy 2015, 16, 329–338.
- Chen, R.; Zhang, H.; Xie, J.; Lin, Y.; Yu, J.; Chen, L. Preparation, lithium storage performance and thermal stability of nickel-rich layered LiNi0.815 Co0.15 Al0.035 O2/RGO composites. ChemElectroChem 2018, 5, 3176–3182.
- Zhang, Q.; Liang, Q.; Nandakumar, D.K.; Qu, H.; Shi, Q.; Alzakia, F.I.; Tay, D.J.J.; Yang, L.; Zhang, X.; Suresh, L.; et al. Shadow enhanced self-charging power system for wave and solar energy harvesting from the ocean. Nat. Commun. 2021, 12, 616.
- Zhang, Q.; Zhang, Z.; Liang, Q.; Shi, Q.; Zhu, M.; Lee, C. All in one, self-powered bionic artificial nerve based on a triboelectric nanogenerator. Adv. Sci. 2021, 8, 2004727.
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418.
- Zhang, Q.; Liang, Q.; Nandakumar, D.K.K.; Ravi, S.K.; Qu, H.; Suresh, L.; Zhang, X.; Zhang, Y.; Yang, L.; Wee, A.T.S.; et al. Energy harvesting from shadow-effect. Energy Environ. Sci. 2020, 13, 2404–2413.
- Zheng, X.; Yan, X.; Sun, Y.; Bai, Z.; Zhang, G.; Shen, Y.; Liang, Q.; Zhang, Y. Au-embedded ZnO/NiO hybrid with excellent electrochemical performance as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 2480–2485.
- Meng, Y.; Nie, C.; Guo, W.; Liu, D.; Chen, Y.; Ju, Z.; Zhuang, Q. Inorganic Cathode Materials for Potassium Ion Batteries. Mater. Today Energy 2022, 25, 100982.
- Lu, S.; Qi, J.; Liu, S.; Zhang, Z.; Wang, Z.; Lin, P.; Liao, Q.; Liang, Q.; Zhang, Y. Piezotronic interface engineering on ZnO/Au-based schottky junction for enhanced photoresponse of a flexible self-powered UV detector. ACS Appl. Mater. Interfaces 2014, 6, 14116–14122.
- Manthiram, A. A reflection on Lithium-Ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550.
- Demirocak, D.E.; Srinivasan, S.S.; Stefanakos, E.K. A review on nanocomposite materials for rechargeable Li-Ion batteries. Appl. Sci. 2017, 7, 731.
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of Lithium Ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630.
- Chaudhary, M.; Tyagi, S.; Gupta, R.K.; Singh, B.P.; Singhal, R. Surface modification of cathode materials for energy storage devices: A review. Surf. Coatings Technol. 2021, 412, 127009.
- Liang, Q.; Zhang, Q.; Gou, J.; Song, T.T.; Arramel; Chen, H.; Yang, M.; Lim, S.X.; Wang, Q.; Zhu, R.; et al. Performance improvement by ozone treatment of 2D PdSe2. ACS Nano 2020, 14, 5668–5677.
- Liang, Q.; Zhang, Q.; Zhao, X.; Liu, M.; Wee, A.T.S. Defect engineering of two-dimensional transition-metal dichalcogenides: Applications, challenges and opportunities. ACS Nano 2021, 15, 2165–2181.
- Li, H.; Zhou, H. Enhancing the performances of Li-Ion batteries by carbon-coating: Present and future. Chem. Commun. 2011, 48, 1201–1217.
- Liang, Q.; Gou, J.; Arramel; Zhang, Q.; Zhang, W.; Wee, A.T.S. Oxygen-induced controllable p-type doping in 2D semiconductor transition metal dichalcogenides. Nano Res. 2020, 13, 3439–3444.
- Inagaki, M. Carbon coating for enhancing the functionalities of materials. Carbon 2011, 50, 3247–3266.
- Su, L.; Jing, Y.; Zhou, Z. Li Ion battery materials with core–shell nanostructures. Nanoscale 2011, 3, 3967–3983.
- Kwon, N.H.; Conder, J.; Srout, M.; Fromm, K.M. Surface modifications of positive-electrode materials for Lithium-Ion batteries. CHIMIA 2019, 73, 880–893.
- Pagot, G.; Bertasi, F.; Nawn, G.; Negro, E.; Delpeuch, A.B.; Vezzù, K.; Cristofori, D.; Di Noto, V. Effect of graphite and copper oxide on the performance of high potential LiPO4 olivine cathodes for Lithium batteries. Electrochim. Acta 2017, 225, 533–542.
- Pagot, G.; Bandiera, M.; Vezzù, K.; Migliori, A.; Bertoncello, R.; Negro, E.; Morandi, V.; Di Noto, V. High valence transition metal-doped olivine cathodes for superior energy and fast cycling lithium batteries. J. Mater. Chem. A 2020, 8, 25727–25738.
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612.
- Zhang, W.-M.; Hu, J.-S.; Guo, Y.-G.; Zheng, S.-F.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in Lithium-Ion batteries. Adv. Mater. 2008, 20, 1160–1165.
- Wu, M.; Xu, B.; Ouyang, C. Physics of electron and lithium-ion transport in electrode materials for Li-Ion batteries. Chin. Phys. B 2016, 25, 018206.
- Yaroslavtsev, A.B.; Stenina, I.A. Carbon coating of electrode materials for lithium-ion batteries. Surf. Innov. 2021, 9, 92–110.
- Liu, Y.; Jiang, L.; Wang, H.; Wang, H.; Jiao, W.; Chen, G.; Zhang, P.; Hui, D.; Jian, X. A brief review for fluorinated carbon: Synthesis, properties and applications. Nanotechnol. Rev. 2019, 8, 573–586.
- Zhang, Y.; Huo, Q.-Y.; Du, P.-P.; Wang, L.-Z.; Zhang, A.-Q.; Song, Y.-H.; Lv, Y.; Li, G.-Y. Advances in new cathode material LiFePO4 for Lithium-Ion batteries. Synth. Met. 2012, 162, 1315–1326.
- Gu, M.; Shi, W.; Zheng, J.; Yan, P.; Zhang, J.-G.; Wang, C. Probing the failure mechanism of nanoscale LiFePO4 for Li-Ion batteries. Appl. Phys. Lett. 2015, 106, 203902.
- Luo, W.-B.; Wen, L.; Luo, H.-Z.; Song, R.-S.; Zhai, Y.-C.; Liu, C.; Li, F. Carbon nanotube-modified LiFePO4 for high rate Lithium Ion batteries. New Carbon Mater. 2014, 29, 287–294.
- Qi, M.; Liu, Y.; Xu, M.; Feng, M.; Gu, J.; Liu, Y.; Wang, L. Improved electrochemical performances of carbon-coated LiFePO4 microspheres for Li-Ion battery cathode. Mater. Res. Express 2019, 6, 115520.
- Fei, H.; Peng, Z.; Yang, Y.; Li, L.; Raji, A.-R.O.; Samuel, E.L.G.; Tour, J.M. LiFePO4 nanoparticles encapsulated in graphene nanoshells for high-performance Lithium-Ion battery cathodes. Chem. Commun. 2014, 50, 7117–7119.
- Su, C.; Bu, X.; Xu, L.; Liu, J.; Zhang, C. A novel LiFePO4/graphene/carbon composite as a performance-improved cathode material for Lithium-Ion batteries. Electrochim. Acta 2012, 64, 190–195.
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358.
- Bao, L.; Li, L.; Xu, G.; Wang, J.; Zhao, R.; Shen, G.; Han, G.; Zhou, S. Olivine LiFePO4 nanocrystallites embedded in carbon-coating matrix for high power Li-Ion batteries. Electrochim. Acta 2016, 222, 685–692.
- Wang, J.; Gu, Y.-J.; Kong, W.-L.; Liu, H.-Q.; Chen, Y.-B.; Liu, W. Effect of carbon coating on the crystal orientation and electrochemical performance of nanocrystalline LiFePO4. Solid State Ion. 2018, 327, 11–17.
- Jiang, G.; Hu, Z.; Xiong, J.; Zhu, X.; Yuan, S. Enhanced performance of LiFePO4 originating from the synergistic effect of graphene modification and carbon coating. J. Alloys Compd. 2018, 767, 528–537.
- Okada, D.; Sugiki, T.; Uematsu, K.; Itadani, A.; Toda, K.; Sato, M.; Yamaguchi, T.; Arimitsu, N.; Nishikawa, S. Electrochemical properties of LiFePO4 cathode materials coated with newly developed carbon black. Electrochemistry 2015, 83, 858–860.
- Pratheeksha, P.M.; Rajeshwari, J.S.; Daniel, P.J.; Rao, T.N.; Anandan, S. Investigation of In situ carbon coated LiFePO4 as a superior cathode material for Lithium-Ion batteries. J. Nanosci. Nanotechnol. 2019, 19, 3002–3011.
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308.
- Yu, F.; Zhang, J.; Wang, C.; Yuan, J.; Yang, Y.; Song, G. Crystal structure and electrochemical performance of lithium-ion battery cathode materials. J. Prog. Chem. 2010, 22, 9–18.
- Lee, M.-J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-Ion batteries. Nano Lett. 2014, 14, 993–999.
- Kraytsberg, A.; Ein-Eli, Y. Higher, stronger, better a review of 5 volt cathode materials for advanced Lithium-Ion batteries. Adv. Energy Mater. 2012, 2, 922–939.
- Jiang, Q.; Wang, X.; Tang, Z. Improving the electrochemical performance of LiMn2O4 by amorphous carbon coating. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 676–679.
- Li, A.; Shao, Z.; Yang, S.; Li, X.; Zhang, A. Precipitation synthesis and enhanced electrochemical performance of graphene-modified LiMn2O4 for Lithium-Ion batteries. Ionics 2020, 26, 3231–3238.
- Cao, J.; Guo, S.; Yan, R.; Zhang, C.; Guo, J.; Zheng, P. Carbon-coated single-crystalline LiMn2O4 nanowires synthesized by high-temperature solid-state reaction with high capacity for Li-Ion battery. J. Alloys Compd. 2018, 741, 1–6.
- Zhang, H.; Zhang, Z.; Zhao, L.; Liu, X.Q. Synthesis and electrochemical performance of spinel LiMn2O4 modified by CNTs. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; Volume 734, pp. 2523–2527.
- Zhuo, H.; Wan, S.; He, C.; Zhang, Q.; Li, C.; Gui, D.; Zhu, C.; Niu, H.; Liu, J. Improved electrochemical performance of spinel LiMn2O4 In Situ coated with graphene-like membrane. J. Power Sources 2014, 247, 721–728.
- Liang, X.; Zeng, S.; Liu, Y.; Shi, L.; Ye, C. Study on carbon black coated LiMn2O4 doped with Cr2O3. High Temp. Mater. Process 2015, 34, 515–518.
- Michalska, M.; Buchberger, D.; Jasiński, J.; Thapa, A.; Jain, A. Surface modification of nanocrystalline LiMn2O4 using graphene oxide flakes. Materials 2021, 14, 4134.
- Park, J.S.; Park, Y.J. Electrochemical performance of carbon coated LiMn2O4 nanoparticles using a new carbon source. J. Electrochem. Sci. Technol. 2016, 7, 139–145.
- Jiang, C.; Tang, Z.; Deng, S.; Hong, Y.; Wang, S.; Zhang, Z. High-performance carbon-coated mesoporous LiMn2O4 cathode materials synthesized from a novel hydrated layered-spinel lithium manganate composite. RSC Adv. 2017, 7, 3746–3751.
- Chudzik, K.; Lis, M.; Świętosławski, M.; Bakierska, M.; Gajewska, M.; Molenda, M. Improving the performance of sulphur doped LiMn2O4 by carbon coating. J. Power Sources 2019, 434, 226725.
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-Ion batteries. J. Power Sources 2020, 460, 228062.
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An overview on the advances of LiCoO2 cathodes for Lithium-Ion batteries. Adv. Energy Mater. 2020, 11, 2000982.
- Qu, Z.; Liu, S.; Zhang, P.; Wang, R.; Wang, H.; He, B.; Gong, Y.; Jin, J.; Li, S. Enhanced eletrochemical performances of LiCoO2 at high cut-off voltage by introducing LiF additive. Solid State Ion. 2021, 365, 115654.
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early Lithium-Battery development. Materials 2020, 13, 1884.
- Kim, J.; Kim, B.; Lee, J.-G.; Cho, J.; Park, B. Direct carbon-black coating on LiCoO2 cathode using surfactant for high-density Li-Ion cell. J. Power Sources 2005, 139, 289–294.
- Cao, Q.; Zhang, H.; Wang, G.; Xia, Q.; Wu, Y.; Wu, H. A novel carbon-coated LiCoO2 as cathode material for lithium ion battery. Electrochem. Commun. 2007, 9, 1228–1232.
- Kwon, N.H. The effect of carbon morphology on the LiCoO2 cathode of Lithium-Ion batteries. Solid State Sci. 2013, 21, 59–65.
- Kwon, N.H.; Yin, H.; Brodard, P.; Sugnaux, C.; Fromm, K.M. Impact of composite structure and morphology on electronic and ionic conductivity of carbon contained LiCoO2 cathode. Electrochim. Acta 2014, 134, 215–221.
- Wang, J. Enhancement of electrochemical performance of LiCoO2 cathode material at high cut-off voltage (4.5 V) by partial surface coating with graphene nanosheets. Int. J. Electrochem. Sci. 2020, 9282–9293.
- Sun, Y.; Dong, H.; Wu, K.; Chen, X.; Wang, S.; Gu, W.; Hong, Z.; Liu, M.; Shen, Y.; Lu, W. Graphene quantum dots coated LiCoO2 for improved cycling stability and thermal safety at high voltage. J. Electroanal. Chem. 2020, 866, 114109.
- Lin, J.; Zeng, C.; Chen, Y.; Lin, X.; Xu, C.; Su, C.-Y. In situ construction of a MOF-derived carbon-encapsulated LiCoO2 heterostructure as a superior cathode for elevated-voltage lithium storage: From experimental to theoretical study. J. Mater. Chem. A 2020, 8, 6607–6618.
- Liang, J.; Sun, Y.; Zhao, Y.; Sun, Q.; Luo, J.; Zhao, F.; Lin, X.; Li, X.; Li, R.; Zhang, L.; et al. Engineering the conductive carbon/PEO interface to stabilize solid polymer electrolytes for all-solid-state high voltage LiCoO2 batteries. J. Mater. Chem. A 2020, 8, 2769–2776.
- Luo, S.; Wang, K.; Wang, J.; Jiang, K.; Li, Q.; Fan, S. Binder-free LiCoO2/carbon nanotube cathodes for high-performance Lithium-Ion batteries. Adv. Mater. 2012, 24, 2294–2298.
- Park, M.S.; Hyun, S.H.; Nam, S.C. Characterization of a LiCoO2 thick film by screen-printing for a Lithium-Ion micro-battery. J. Power Sources 2006, 159, 1416–1421.
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and calcination of high-capacity LiNiO2 cathode material for Lithium-Ion batteries. Appl. Sci. 2020, 10, 8988.
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and back again—The journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 2018, 58, 10434–10458.
- Murali, N.; Veeraiah, V. Preparation, dielectric and conductivity studies of LiNi1-XMgxO2 cathode materials for Lithium-Ion batteries. Process. Appl. Ceram. 2017, 11, 258–264.
- Vandenberg, A.; Hintennach, A. A comparative microwave-assisted synthesis of carbon-coated LiCoO2 and LiNiO2 for Lithium-Ion batteries. Russ. J. Electrochem. 2015, 51, 310–317.
- Li, C.; Zhang, H.; Fu, L.; Liu, H.; Wu, Y.; Rahm, E.; Holze, R.; Wu, H. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883.
- Jung, C.-H.; Shim, H.; Eum, D.; Hong, S.-H. Challenges and recent progress in LiNixCoyMn1−x−yO2 (NCM) cathodes for Lithium-Ion batteries. J. Korean Ceram. Soc. 2020, 58, 1–27.
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291.
- Kim, H.-J.; Krishna, T.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A comprehensive review of Li-Ion battery materials and their recycling techniques. Electronics 2020, 9, 1161.
- Chen, G.; Peng, B.; Han, R.; Chen, N.; Wang, Z.; Wang, Q. A robust carbon coating strategy toward Ni-rich lithium cathodes. Ceram. Int. 2020, 46, 20985–20992.
- Li, X.; Zhao, X.; Wang, M.-S.; Zhang, K.-J.; Huang, Y.; Qu, M.-Z.; Yu, Z.-L.; Geng, D.-S.; Zhao, W.-G.; Zheng, J.-M. Improved rate capability of a LiNi1/3Co1/3Mn1/3O2/CNT/graphene hybrid material for Li-Ion batteries. RSC Adv. 2017, 7, 24359–24367.
- Hou, Q.; Cao, G.; Wang, P.; Zhao, D.; Cui, X.; Li, S.; Li, C. Carbon coating nanostructured-LiNi1/3Co1/3Mn1/3O2 cathode material synthesized by chemical vapor deposition method for high performance lithium-ion batteries. J. Alloys Compd. 2018, 747, 796–802.
- Yang, S.; Fan, Q.; Shi, Z.; Liu, L.; Liu, J.; Ke, X.; Liu, J.; Hong, C.; Yang, Y.; Guo, Z. Superior stability secured by a four-phase cathode electrolyte interface on a Ni-rich cathode for Lithium-Ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 36742–36750.
- Yang, C.; Zhang, X.; Huang, M.; Huang, J.; Fang, Z. Preparation and rate capability of carbon coated LiNi1/3Co1/3Mn1/3O2 as cathode material in Lithium-Ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415.
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of Lithium-Ion batteries. Sci. Rep. 2020, 10, 11114.
- Ban, C.; Li, Z.; Wu, Z.; Kirkham, M.J.; Chen, L.; Jung, Y.S.; Payzant, E.A.; Yan, Y.; Whittingham, M.S.; Dillon, A.C. Extremely durable high-rate capability of a LiNi0.4Mn0.4Co0.2O2 cathode enabled with single-walled carbon nanotubes. Adv. Energy Mater. 2010, 1, 58–62.
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in Lithium-Ion batteries. J. Power Sources 2017, 352, 187–193.
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.-G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561.
- Nguyen, V.H.; Ngo, M.D.; Kim, Y.H. Effect of soybean oil as a carbon source on the electrochemical property of LiNi1/3Co1/3Mn1/3O2 cathode material for Lithium Ion battery. Carbon Lett. 2020, 30, 621–626.
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S.; Bo, D. Recent progress in capacity enhancement of LiFePO4 cathode for Li-Ion batteries. J. Electrochem. Energy Convers. Storage 2020, 18, 1–54.
- Purwanto, A.; Yudha, C.S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 2018, 5, 122001.
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2021, 238, 121652.
- Wang, D.; Liu, W.; Zhang, X.; Huang, Y.; Xu, M.; Xiao, W. Review of modified nickel-cobalt lithium aluminate cathode materials for Lithium-Ion batteries. Int. J. Photoenergy 2019, 2019, 2730849.
- Fang, R.; Miao, C.; Nie, Y.; Wang, D.; Xiao, W.; Xu, M.; Wang, C. Degradation mechanism and performance enhancement strategies of LiNixCoyAl1-x-yO2 (x≥0.8) cathodes for rechargeable Lithium-Ion batteries: A review. Ionics 2020, 26, 3199–3214.
- Visbal, H.; Aihara, Y.; Ito, S.; Watanabe, T.; Park, Y.; Doo, S. The effect of diamond-like carbon coating on LiNi0.8Co0.15Al0.05O2 particles for all solid-state lithium-ion batteries based on Li2S–P2S5 glass-ceramics. J. Power Sources 2016, 314, 85–92.
- Yu, J.; Li, H.; Zhang, G.; Li, X.; Huang, J.; Li, C.; Wei, C.; Xiao, C. Carbon nanotubes coating on LiNi0.8Co0.15Al0.05O2 as cathode materials for Lithium battery. Int. J. Electrochem. Sci. 2017, 12, 11892–11903.
- Gao, P.; Jiang, Y.; Zhu, Y.; Hu, H. Improved cycle performance of nitrogen and phosphorus co-doped carbon coatings on lithium nickel cobalt aluminum oxide battery material. J. Mater. Sci. 2018, 53, 9662–9673.
- Li, Y.; Yu, H.; Hu, Y.; Jiang, H.; Li, C. Surface-engineering of layered LiNi0.815Co0.15Al0.035O2 cathode material for high-energy and stable Li-Ion batteries. J. Energy Chem. 2018, 27, 559–564.
- Liu, Z.; Wang, Z.; Lu, T.; Dai, P.; Gao, P.; Zhu, Y. Modification of LiNi0.8Co0.15Al0.05O2 using nanoscale carbon coating. J. Alloys Compd. 2018, 763, 701–710.
- Lim, J.-M.; Luu, N.S.; Park, K.-Y.; Tan, M.T.Z.; Kim, S.; Downing, J.R.; He, K.; Dravid, V.P.; Hersam, M.C. Enhancing nanostructured Nickel-rich Lithium-Ion battery cathodes via surface stabilization. J. Vac. Sci. Technol. A 2020, 38, 063210.
- Park, K.; Lim, J.; Luu, N.S.; Downing, J.R.; Wallace, S.G.; Chaney, L.E.; Yoo, H.; Hyun, W.J.; Kim, H.; Hersam, M.C. Concurrently approaching volumetric and specific capacity limits of Lithium battery cathodes via conformal pickering emulsion graphene coatings. Adv. Energy Mater. 2020, 10, 2001216.
- Zhao, H.; Law, H.; Liao, S.; Chen, D.; Lin, P. Novel graphitic sheets with ripple-like folds as an NCA cathode coating layer for high-energy-density Lithium-Ion batteries. Nanotechnology 2020, 32, 08LT01.
- Feng, D.; Liu, Q.; Hu, T.; Chen, Y.; Zeng, T. Boosting cyclability performance of the LiNi0.8Co0.15Al0.05O2 cathode by a polyacrylonitrile-induced conductive carbon surface coating. Ceram. Int. 2021, 47, 12706–12715.
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene collage on Ni-rich layered oxide cathodes for advanced Lithium-Ion batteries. Nat. Commun. 2021, 12, 2145.
- Yang, H.; Wang, Y.; Duh, J.-G. Developing a diamine-assisted polymerization method to synthesize nano-LiMnPO4 with n-doped carbon from polyamides for high-performance Li-Ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 13302–13311.
- Chen, X.; Ma, F.; Li, Y.; Liang, J.; Matthews, B.; Sokolowski, J.; Han, J.; Wu, G.; Lu, X.; Li, Q. Nitrogen-doped carbon coated LiNi0.6Co0.2Mn0.2O2 cathode with enhanced electrochemical performance for Li-Ion batteries. Electrochim. Acta 2018, 284, 526–533.
This entry is offline, you can click here to edit this entry!
