Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Despite the noticeable evolution in wound treatment over the centuries, a functional material that promotes correct and swift wound healing is important, considering the relative weight of chronic wounds in healthcare. Difficult to heal in a fashionable time, chronic wounds are more prone to infections and complications thereof. Hydrogels are highly hydrated cross-linked polymers arranged in a matrix-like fashion that allow significant water retention (over 90% of their dry weight) in their three-dimensional network.
- hydrogel
- chronic wounds
- NO
1. Introduction—How Wound Care Is Still Relevant Nowadays
Wounds are ruptured and therefore structurally and physiologically compromised skin, caused by either trauma or physiological conditions. Depending on the time required to heal, wounds are typically categorized into acute or chronic [1]. Acute wounds tend to heal relatively fast, while chronic wounds take longer to properly heal. The latter most often arise from complications of specific diseases, with ulcers being the most common type of long-term wounds. Unfortunately, reoccurrence is a major issue in disease-caused chronic wounds, and it can only be avoided by the cure or management of the underlying disease [2]. Due to their high propensity to reappear, chronic wounds burden the healthcare system, and most importantly, negatively impact the quality of life of patients [3][4].
Chronic wounds (i.e., venous, arterial, pressure, and diabetic ulcers) have distinct causes but some characteristics in common, namely, infection, prolonged inflammatory phase, and biofilm formation. Cell abnormalities are also observed in chronic wounds, such as decreased growth factor receptors and reduced mitogenic potential, which impair cells from reacting to environmental signals [5]. These long-term wounds are more susceptible to infection, which further delays wound healing [6], and if left untreated, can cause impaired mobility, limb amputation, and eventually lead to death [7]. A study on the incidence of healthcare-associated infections in European Union countries, including Iceland, Norway, and the United Kingdom, during the period 2016–2017, showed that these infections easily exceeded 8 million per year, and over 3 million patients attained such infections each year at acute care hospitals [8]. Antimicrobial-resistant infections are responsible for large numbers of deaths (ca., 30,000 Europeans in 2020), and have been overshadowed by the still active coronavirus disease pandemic [9]. For further information regarding chronic wounds, reviews on this subject are recommend [4][5][7].
Wound healing is an elaborate cascade-like process that has four complex and overlapping phases (i.e., hemostasis, inflammation, proliferation, and remodeling [10]) that begin immediately after injury and last until re-epithelialization of the skin is completed [6][7] (Figure 1). Hemostasis starts immediately after injury and lasts between a few minutes to an hour. During this stage, platelets arrive to the wound bed, adhere to the extracellular matrix (ECM), and secrete proteins that initiate fibrin production and deposition, thus creating a clot that interrupts the bleeding. Throughout the process, these platelets also produce growth factors that attract neutrophils, macrophages, and fibroblasts to the wound bed. The next phase, inflammation, lasts for ca. 3 days, during which inflammation mediators increase the permeability of blood vessels and facilitate the arrival of neutrophils to the site. Neutrophils digest pathogens, foreign material, damaged cells, and ECM through phagocytosis. Once monocytes arrive and differentiate to macrophages, these instigate the proliferation of fibroblasts, smooth-muscle cells, and endothelial cells, hence beginning the proliferation stage. Starting around 48 h after injury, this phase consists of fibroblast proliferation, collagen and other ECM components production and deposition, and angiogenesis. The last phase, remodeling, starts 2–3 weeks after injury and can take several months to be completed. The ECM produced during the proliferation stage is remodeled by enzymes produced by fibroblasts, and lastly, macrophages and fibroblasts depart the wound site, ceasing the inflammation and proliferation stages [1][7].
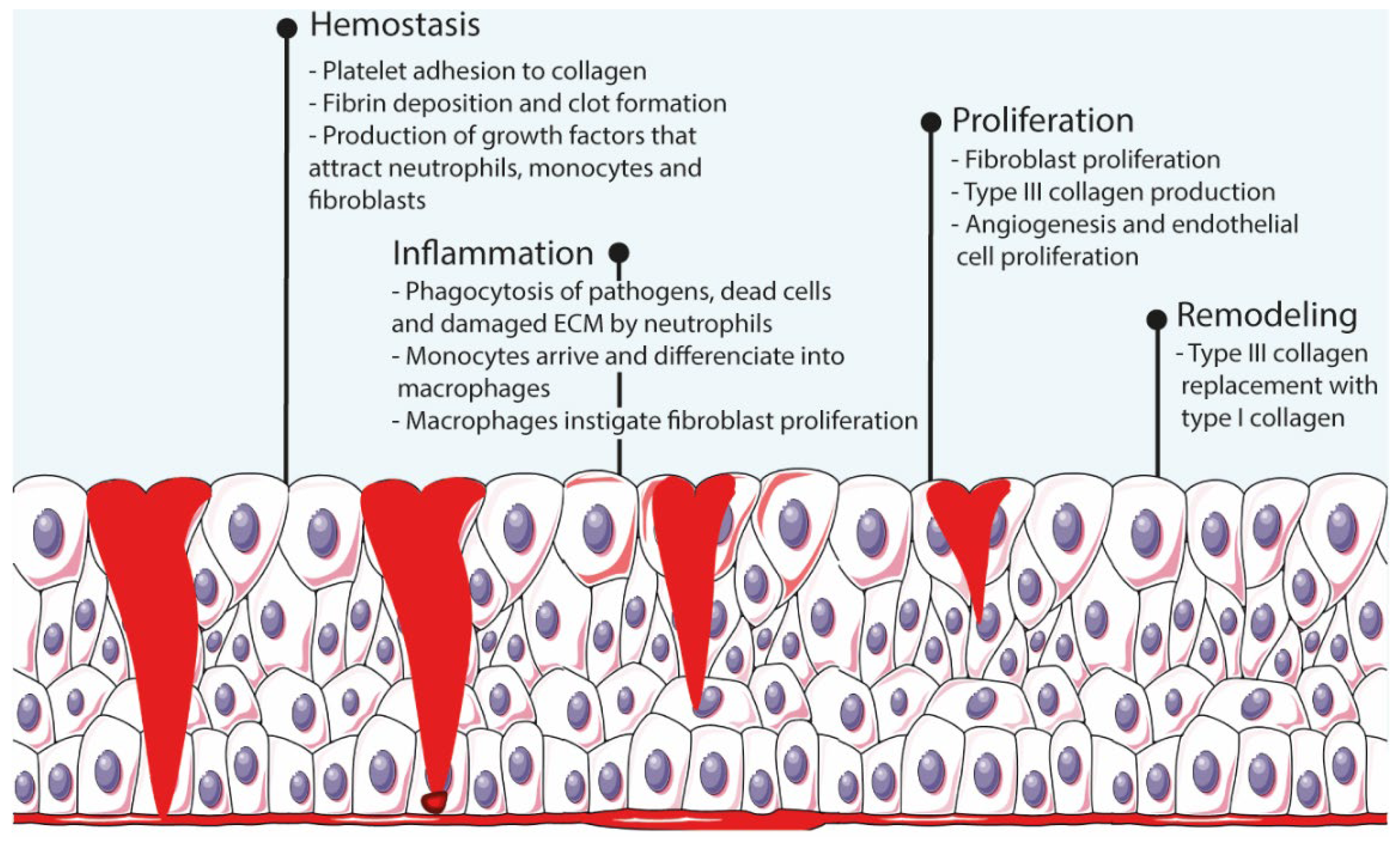
Figure 1. Schematic representation of the wound healing process.
Any imbalance in the process leads to impaired healing and chronic wound development. For instance, bacteria propagation enhances the inflammatory response and deeply compromises angiogenesis, thus impacting the amount of oxygen and nutrients capable of reaching the wound bed [10]. The evolution to chronic wounds can be prevented though a simple wound care regiment. Wound care is essentially performed in two steps: debridement and wound coverage. Debridement is the cleaning of the wound through removal of tissue debris that would otherwise be fuel for microbial proliferation and allows the exposure of healthy tissue to facilitate its proliferation [4][11]. The next step is the physical protection of the wound through the application of wound dressings.
Wound dressings are materials designed to cover damaged skin and are primarily meant to promote the wound healing process by acting as a pathogen penetration barrier while keeping the wound site moist through the absorption of excess exudate [12][13][14]. Moisture retention contributes to a proper and swift wound closure process as it aids the migration of new skin cells [4][15]. Deficient or excess exudate absorption is detrimental for proper wound healing as it leads to a microbial-friendly environment or dry wound site, respectively. Exudate levels differ during the healing stages, with high levels for the first 48 h [16]; thus, the main guideline is that wound dressings should ideally only absorb excess exudate without compromising the healing process [4]. Comparatively to normal skin, water loss is higher in wounds, reaching up to 5000 g·m−2d−1, around 20-fold the water loss of normal skin when at 35 °C. It has been disclosed that wound healing benefits from water vapor transmission values of around 2000–2500 g·m−2d−1 [17].
Since traditional wound dressings implicate constant reapplication and complementary methods to keep the wound aseptic, the development of wound dressings with attributes relevant for wound healing has been encouraged. Most research focuses on infection prevention, but besides antimicrobial properties, wound dressings can also be complemented with drugs or other components that accelerate the healing process, such as growth factors [18], anti-inflammatory drugs, and cytokines [19]. Growth factors, which are at low levels in chronic wounds, contribute to wound healing through the performance of several functions, including chemoattraction of macrophages, fibroblasts, and other cells; angiogenesis; and proliferation of fibroblasts and endothelial cells [18]. Cytokines have a role in wound healing and are responsible for inducing the migration of immune system cells to the wound site. Studies on the delivery of growth factors and cytokines showed increased wound closure [19].
For the past few decades, efforts have been made to confer antimicrobial properties to wound dressing supports such as foams, sponges, hydrogels, and gauzes. The simplest route is to load antimicrobial agents (e.g., silver [4], antibiotics, quaternary ammonium, and metallic nanoparticles [3]) into porous materials, but the materials can display antibacterial activity on their own (e.g., chitosan has been proven to have antibacterial activity [20]). Performance limitations of textile wound dressings (e.g., cotton or wool) have also fueled the search for different materials. Although soft in texture [3], textile wound dressings are devoid of the flexibility required for wounds located in mobility-related body parts such as joints [21]. When applied to burns, textile wound dressings adhere to the wound site in an uncontrollable manner, making its removal painful as the superficial layer of the wound bed is stripped in the process [1][22]. The ultimate wound dressings should be flexible [13], have antibacterial or at least bacteriostatic activity [21], exhibit adequate exudate absorption and gas permeability [13], allow a pain-free removal for the greater comfort possible, and be biocompatible [22][23][24][25]. The biocompatibility of a material is ascertained after an extensive array of tests that study the physical, chemical, and mechanical properties of the material as well as the potential adverse effects (i.e., allergenic, mutagenic, and cytotoxic) that may occur from its use, being crucial that the material does not elicit substantial damages or toxic effects to the body [26]. Research has shown that effective wound dressings exhibit porosity between 50% and 60%; these high values allow the transfer of oxygen and nutrients to the wound bed cells in contact with the dressing [17][23]. Pore size is also important since small pores physically hinder bacteria from reaching the wound site [11]. The interest is set on novel materials that intrinsically have a considerable number of desired properties (e.g., inherent antimicrobial activity and biocompatibility) and can perform well in wound environments. NO is a promising component regarding the design of ideal wound dressings. Due to its diminutive dimensions, this radical easily penetrates porous materials to reach the wound bed and triggers death cell mechanisms once it reaches bacterial membranes [27]. However, as a bioactive agent, NO demands storage and delivery vehicles. Therefore, it is theorized that hydrogels as vectors for the delivery of NO can be designed to fit the requirements of an excellent wound dressing. Polymers can form an assortment of materials that can accommodate the requirements of ideal wound dressings (e.g., foams, sponges, fibers and hydrogels), and due to a matrix similar to the extracellular matrix, nanofibers and hydrogels are the most explored polymeric materials for wound healing purposes [28]. The differentiating factor of NO-releasing hydrogels for wound healing is the functionality of NO. Antibacterial wound dressings on the market have an antimicrobial agent whose functionality is limited to preventing infections. NO, however, is unique because it participates in multiple aspects with regard to the evolution of the healing process besides infection prevention.
2. Hydrogels
Hydrogels are highly hydrated cross-linked polymers arranged in a matrix-like fashion that allow significant water retention (over 90% of their dry weight) in their three-dimensional network [29]. The most common natural polymers used for hydrogel formulation include collagen, alginate, hyaluronic acid [20], gelatin [30], cellulose [31], and chitosan [32]. Since most natural polymers already display biocompatibility and biodegradability [26], their high bioavailability further consolidates the proportion of interest in biopolymers over the past few decades [6]. However, biomedical applications are not exclusively reliant on natural polymers since many synthetic polymers are well-established biocompatible polymers (e.g., Poly(ethylene glycol) (PEG) [33] and Pluronic F-127 [34]) and are widely used for biomedical applications. Unlike natural polymers, synthetic polymers allow a greater degree of control over their composition. Biopolymers, however, require purification, and homogeneity is sometimes difficult to achieve due to different sources.
The structure and properties of hydrogels make them promising materials for the design of transdermal or injectable drug delivery systems, wound dressings, and adhesives [30][35]. Hydrogels are materials of great interest for wound healing due to their flexibility, adhesion, stability, and biodegradability, in addition to the capability of maintaining the wounded site moist, which helps to accelerate the healing rate [20][21][31][36]. Their porous extracellular matrix-like structure is also an important aspect to consider as it can facilitate the absorption of exudate from the wound bed [21][37] (Figure 2). Hydrogels can be modified to improve desired properties. For instance, knowing that hydrogel-tissue adhesion is limited in extreme wet conditions (e.g., bleeding), it was developed hemostatic hydrogels with enhanced tissue adhesion by grafting molecules that mimic adhesive components found in nature, namely methacrylate and dopamine [38].
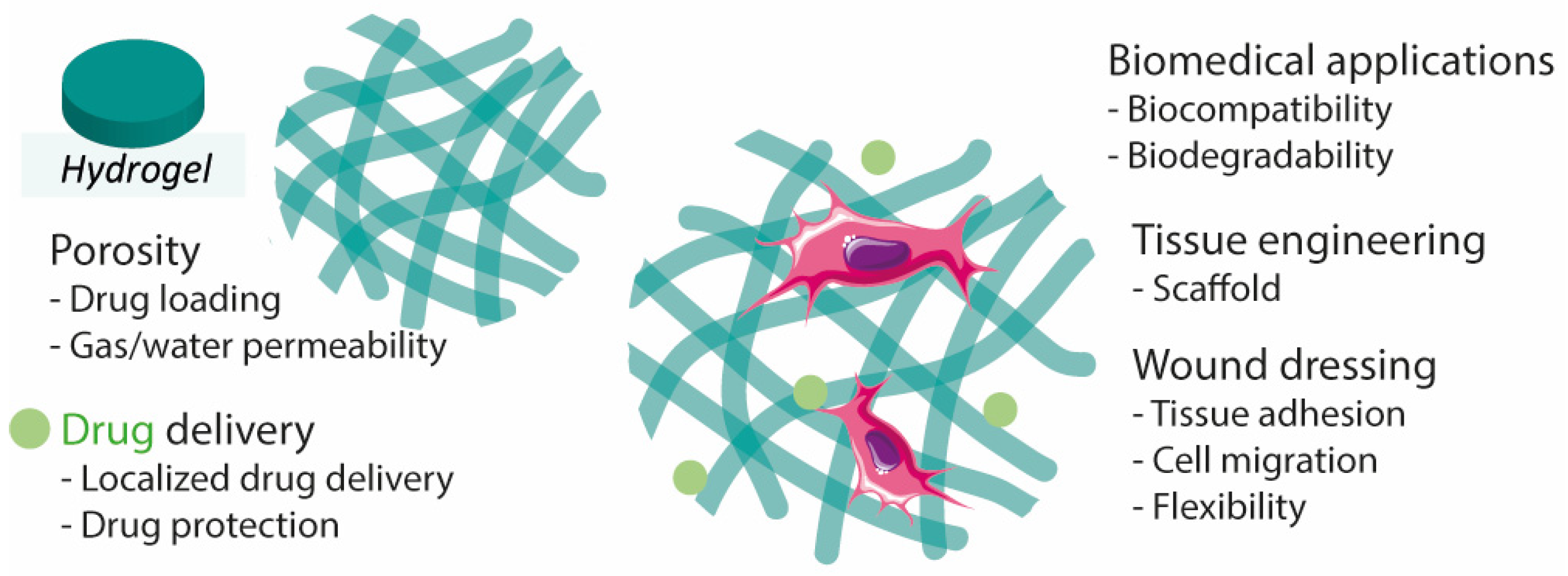
Figure 2. Hydrogel characteristics for wound healing, drug delivery, and tissue engineering.
Due to the characteristics mentioned above, hydrogels offer the possibility to simultaneously perform two functions, namely as a drug (or any bioactive agent relevant to wound closure) delivery system, and as a wound dressing [21]. In addition, hydrogels can be implemented as film/membranes [39], as a powder [40] (particles that gel in contact with liquid), or even be formed in situ (injectable [10][41][42][43]), making this class of materials highly convenient. For instance, hydrogel-forming powders better adapt to irregular wounds, and injectable hydrogels are excellent candidates for wounds located in mobility-related places [40][44]. In addition, powdering hydrogels has been reported as a route to patch and/or recycle mechanically damaged hydrogels. Powdered self-healing hydrogels regained their initial mechanical properties upon hydration [45].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14071377
References
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336.
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82.
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-inspired immobilization of zwitterionic silver nanoparticles toward antibacterial cotton gauze for promoting wound healing. Chem. Eng. J. 2021, 409, 128291.
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610.
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314.
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; Fakhry, H.; Ajarem, J.; Maodaa, S.N.; Allam, A.A.; Hafez, E.E. Wound dressing of chitosan-based-crosslinked gelatin/ polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr. Polym. 2021, 255, 117484.
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166.
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516.
- Monnet, D.L.; Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Eurosurveillance 2020, 25, 2001886.
- Cheng, W.; Wang, M.; Chen, M.; Niu, W.; Li, Y.; Wang, Y.; Luo, M.; Xie, C.; Leng, T.; Lei, B. Injectable antibacterial antiinflammatory molecular hybrid hydrogel dressing for rapid MDRB-infected wound repair and therapy. Chem. Eng. J. 2021, 409, 128140.
- Ketabchi, N.; Dinarvand, R.; Adabi, M.; Gholami, M.; Firoozi, S.; Amanzadi, B.; Faridi-Majidi, R. Study of Third-Degree Burn Wounds Debridement and Treatment by Actinidin Enzyme Immobilized on Electrospun Chitosan/PEO Nanofibers in Rats. Biointerface Res. Appl. Chem. 2021, 11, 10358–10370.
- Matsliah, L.; Goder, D.; Giladi, S.; Zilberman, M. In vitro characterization of novel multidrug-eluting soy protein wound dressings. J. Biomater. Appl. 2021, 35, 978–993.
- Karakaya, P.S.; Oktay, A.; Seventekin, N.; Yesil-Celiktas, O. Design of a new generation wound dressing with pine bark extract. J. Ind. Text. 2021, 50, 1193–1204.
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311.
- Hajikhani, M.; Emam-Djomeh, Z.; Askari, G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021, 172, 143–153.
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171.
- Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers 2017, 9, 727.
- Park, J.W.; Hwang, S.R.; Yoon, I.-S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259.
- Jimi, S.; Jaguparov, A.; Nurkesh, A.; Sultankulov, B.; Saparov, A. Sequential Delivery of Cryogel Released Growth Factors and Cytokines Accelerates Wound Healing and Improves Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 345.
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839.
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199.
- Montaser, A.S.; Rehan, M.; El-Senousy, W.M.; Zaghloul, S. Designing strategy for coating cotton gauze fabrics and its application in wound healing. Carbohydr. Polym. 2020, 244, 116479.
- Mirmajidi, T.; Chogan, F.; Rezayan, A.H.; Sharifi, A.M. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int. J. Pharm. 2021, 596, 120213.
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101.
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256, 117590.
- Porto, I.C.C.M. Polymer Biocompatibility. In Polymerization; Gomes, A.S., Ed.; IntechOpen: London, UK, 2012.
- Jeong, H.; Kim, T.; Earmme, T.; Hong, J. Acceleration of Nitric Oxide Release in Multilayer Nanofilms through Cu(II) Ion Intercalation for Antibacterial Applications. Biomacromolecules 2021, 22, 1312–1322.
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144.
- Champeau, M.; Seabra, A.B.; de Oliveira, M.G. Hydrogels for Topical Nitric Oxide Delivery. In Nitric Oxide Donors: Novel Biomedical Applications and Perspectives; Seabra, A.B., Ed.; Elsevier: London, UK, 2017; pp. 313–330.
- Pal, A.; Bajpai, J.; Bajpai, A.K. Poly (acrylic acid) grafted gelatin nanocarriers as swelling controlled drug delivery system for optimized release of paclitaxel from modified gelatin. J. Drug Deliv. Sci. Technol. 2018, 45, 323–333.
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811.
- Ling, Z.; Chen, Z.; Deng, J.; Wang, Y.; Yuan, B.; Yang, X.; Lin, H.; Cao, J.; Zhu, X.; Zhang, X. A novel self-healing polydopamine-functionalized chitosan-arginine hydrogel with enhanced angiogenic and antibacterial activities for accelerating skin wound healing. Chem. Eng. J. 2021, 420, 130302.
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298.
- Pelegrino, M.T.; Lima, B.D.; do Nascimento, M.H.M.; Lombello, C.B.; Brocchi, M.; Seabra, A.B. Biocompatible and Antibacterial Nitric Oxide-Releasing Pluronic F-127/Chitosan Hydrogel for Topical Applications. Polymers 2018, 10, 452.
- Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Recent progress of nanocarrier-based therapy for solid malignancies. Cancers 2020, 12, 2783.
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826.
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351.
- Han, W.; Zhou, B.; Yang, K.; Xiong, X.; Luan, S.; Wang, Y.; Xu, Z.; Lei, P.; Luo, Z.; Gao, J.; et al. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020, 5, 768–778.
- Lourenco, S.D.; de Oliveira, M.G. Topical photochemical nitric oxide release from porous poly(vinyl alcohol) membrane for visible light modulation of dermal vasodilation. J. Photochem. Photobiol. A Chem. 2017, 346, 548–558.
- Lee, J.; Hlaing, S.P.; Cao, J.F.; Hasan, N.; Ahn, H.J.; Song, K.W.; Yoo, J.W. In Situ Hydrogel-Forming/Nitric Oxide-Releasing Wound Dressing for Enhanced Antibacterial Activity and Healing in Mice with Infected Wounds. Pharmaceutics 2019, 11, 496.
- Yang, Y.; Zhou, Y.T.; Li, Y.L.; Guo, L.Y.; Zhou, J.; Chen, J.H. Injectable and self-healing hydrogel containing nitric oxide donor for enhanced antibacterial activity. React. Funct. Polym. 2021, 166, 10.
- Liu, H.L.; Zhu, X.L.; Guo, H.M.; Huang, H.L.; Huang, S.H.; Huang, S.S.; Xue, W.; Zhu, P.; Guo, R. Nitric oxide released injectable hydrogel combined with synergistic photothermal therapy for antibacterial and accelerated wound healing. Appl. Mater. Today 2020, 20, 12.
- Joseph, C.A.; McCarthy, C.W.; Tyo, A.G.; Hubbard, K.R.; Fisher, H.C.; Altscheffel, J.A.; He, W.L.; Pinnaratip, R.; Liu, Y.; Lee, B.P.; et al. Development of an Injectable Nitric Oxide Releasing Poly(ethylene) Glycol-Fibrin Adhesive Hydrogel. ACS Biomater. Sci. Eng. 2019, 5, 959–969.
- Friedman, A.J.; Han, G.; Navati, M.S.; Chacko, M.; Gunther, L.; Alfieri, A.; Friedman, J.M. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008, 19, 12–20.
- Miao, H.; Hao, W.; Liu, H.; Liu, Y.; Fu, X.; Huang, H.; Ge, M.; Qian, Y. Highly Flexibility, Powder Self-Healing, and Recyclable Natural Polymer Hydrogels. Gels 2022, 8, 89.
This entry is offline, you can click here to edit this entry!
