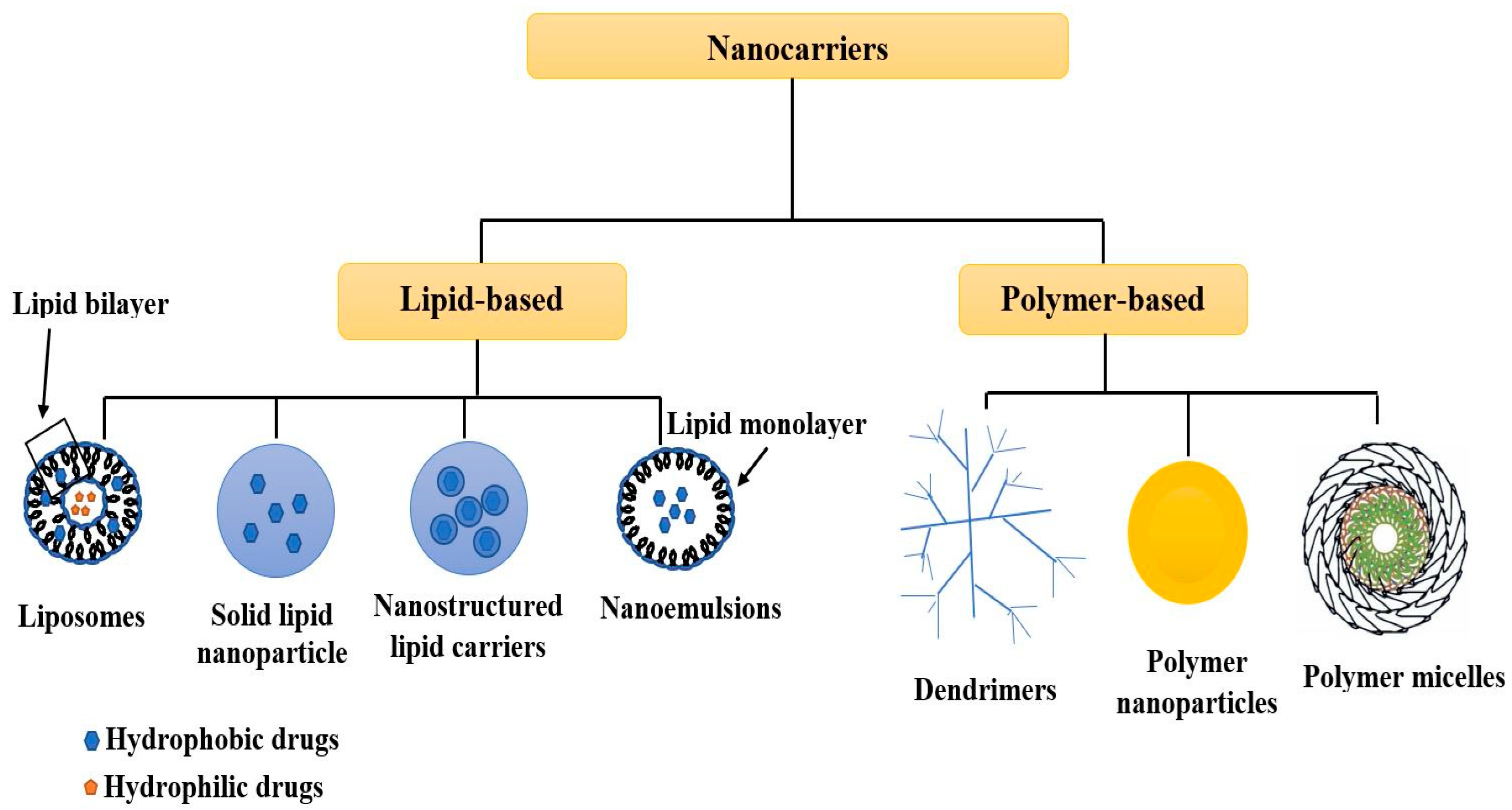
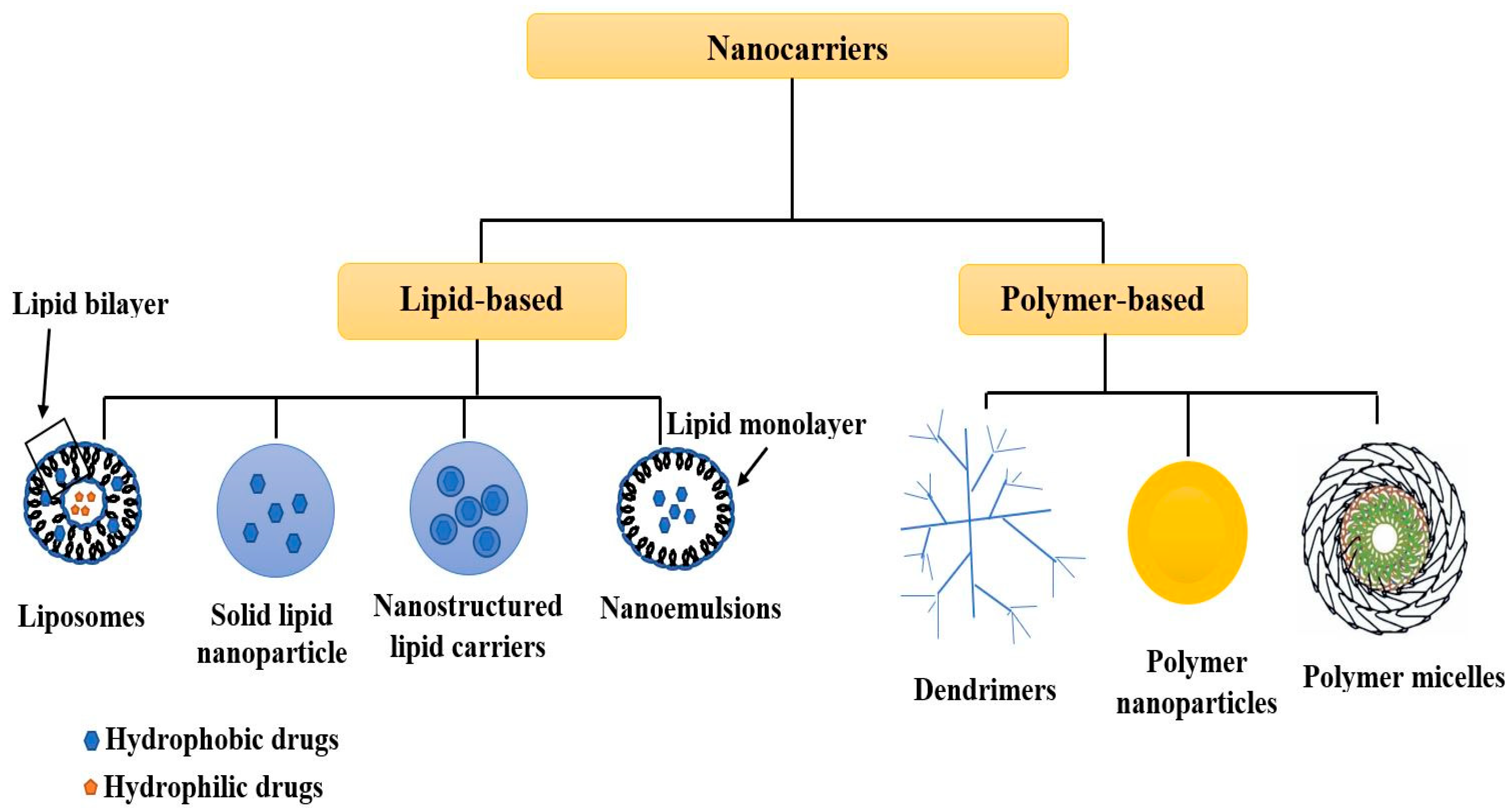
The synthesis of tailored and highly engineered multifunctional pharmaceutical nanocarriers is an emerging field of study in drug delivery applications. They have a high surface-area-to-volume ratio, aiding the targeted drug’s bio-distribution and pharmacokinetic properties. Therefore, the characterization of nanocarriers is critical for understanding their physicochemical properties, which significantly impact their molecular and systemic functioning. To achieve specific goals, particle size, surface characteristics, and drug release properties of nanocarriers must be managed. An overview of the applications of non-destructive testing techniques (NDTT) to reveal the characteristics of nanocarriers, considering their surface charge, porosity, size, morphology, and crystalline organization. The compositional and microstructural characterization of nanocarriers through NDTT, such as dynamic light scattering, X-ray diffraction, confocal laser scanning microscopy, ultraviolet-visible spectroscopy, scanning electron microscopy, atomic force microscopy, and nuclear magnetic resonance spectroscopy, have been comprehensively reviewed. Furthermore, NDTT is only used to characterize physicochemical parameters related to the physiological performance of nanocarriers but does not account for nanocarrier toxicity. Hence, it is highly recommended that in the future, NDTT be developed to assess the toxicity of nanocarriers. In addition, by developing more advanced, effective, and precise techniques, such as machine vision techniques using artificial intelligence, the future of using NDTT for nanocarrier characterization will improve the evaluation of internal quality parameters.
- drug delivery
- non-destructive testing
- NDTT
1. Introduction
| Technique | Nanocarriers Examined | Parameters Examined | References |
|---|---|---|---|
| Scanning electron microscopy | Mesoporous silica materials | Structure/shape, size distribution, and particle size | [16] |
| Scanning electron microscopy | Core-shell nanoparticles of magnetic Fe3O4-poly (N-isopropylacrylamide) grafted with chitosan | Structure/shape, size distribution, and particle size | [17] |
| Scanning electron microscopy | Ascorbic acid-modified chitosan based superparamagnetic iron oxide nanoparticles | Identification of elemental composition, structure/shape, size distribution, and particle size | [18] |
| Dynamic light scattering | Gelatin nanoparticles | Size distribution, particle size, and surface charge | [19] |
| Dynamic light scattering | Liposomes consisting of phosphatidylcholine or dimyristoylphosphatidylcholine | Size distribution and particle size | [20] |
| Dynamic light scattering | 5-Fluorouracil and Carmofur loaded polyethylene glycol/rosin ester nanocarriers | Size distribution and particle size | [21] |
| X-ray diffraction | Graphene oxide/Fe3O4 nanocomposite | Phase and structure | [22] |
| X-ray diffraction | Pluronic F127 coated magnetic silica nanocarriers | Crystalline phase and structure | [23] |
| X-ray diffraction | Camptothecin-loaded holmium ferrite nanocarrier | Phase and structure | [24] |
| Atomic force microscopy | Silica nanoparticle | Aggregation topography, Size, shape, and structure | [25] |
| Atomic force microscopy | Chitosan-gum arabic embedded alizarin nanocarriers | Surface topography and uniformity | [26] |
| Atomic force microscopy | Glucosamine-conjugated graphene quantum dots | Surface topography and morphology | [27] |
| Confocal laser scanning microscopy | Polystyrene nanoparticles | Distribution, size, and shape | [28] |
| Confocal laser scanning microscopy | Liposomes | Effect of penetration ability and distribution | [29] |
| Confocal laser scanning microscopy | Miconazole based on chitosan-coated iron oxide nanoparticles | Structure/shape | [30] |
| Nuclear magnetic resonance spectroscopy | Starch nanoparticles | Purity, structure, and composition | [31] |
| Nuclear magnetic resonance spectroscopy | Dehydropeptide nanocarrier | Molecular conformation | [32] |
| Nuclear magnetic resonance spectroscopy | Poly (glycidyl methacrylate)-based double-shell magnetic nanocarrier | Structure and composition | [33] |
2. Non-Destructive Testing Techniques
2.1. X-ray Diffraction
2.1.1. Working Principle
2.1.2. Application
2.2. Ultraviolet-Visible Spectroscopy
2.2.1. Working Principle
2.2.2. Application
2.3. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
2.3.1. Working Principle
2.3.2. Application
This entry is adapted from the peer-reviewed paper 10.3390/biophysica2030016
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Senthilkumar, N.; Sharma, P.K.; Sood, N.; Bhalla, N. Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord. Chem. Rev. 2021, 445, 214082.
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Junior, J.A.O.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991.
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235.
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267.
- Nazila, K.; Yameen, B.; Wu, J.; Farokhzad, O. Nanoparticles: Mechanisms of controlling drug release Nazila. Chem. Rev. 2016, 116, 2602–2663.
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019.
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161.
- Bailey, C.M.; Kamaloo, E.; Waterman, K.L.; Wang, K.F.; Nagarajan, R.; Camesano, T.A. Size dependence of gold nanoparticle interactions with a supported lipid bilayer: A QCM-D study. Biophys. Chem. 2015, 203, 51–61.
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539.
- Reich, G. Near-infrared spectroscopy and imaging: Basic principles and pharmaceutical applications. Adv. Drug Deliv. Rev. 2005, 57, 1109–1143.
- Awotwe-Otoo, D.; Zidan, A.S.; Rahman, Z.; Habib, M.J. Evaluation of anticancer drug-loaded nanoparticle characteristics by nondestructive methodologies. AAPS PharmSciTech 2012, 13, 611–622.
- Gowen, A.; O’donnell, C.; Cullen, P.J.; Bell, S. Recent applications of chemical imaging to pharmaceutical process monitoring and quality control. Eur. J. Pharm. Biopharm. 2008, 69, 10–22.
- Rahman, Z.; Zidan, A.S.; Khan, M.A. Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2010, 76, 127–137.
- Lim, M.K.; Cao, H. Combining multiple NDT methods to improve testing effectiveness. Constr. Build. Mater. 2013, 38, 1310–1315.
- Ilavarasi Jeyamalar, J.; Krishnaveni, M.; Kannan, C. Synthesis and characterization of Ni-incorporated mesoporous silica material for its potential applications in oligomerization of glycerol. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1–7.
- Moradi, S.; Najjar, R.; Hamishehkar, H.; Lotfi, A. Triple-responsive drug nanocarrier: Magnetic core-shell nanoparticles of Fe3O4@poly(N-isopropylacrylamide)-grafted-chitosan, synthesis and in vitro cytotoxicity evaluation against human lung and breast cancer cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103426.
- Karimi Jabali, M.; Allafchian, A.R.; Jalali, S.A.H.; Shakeripour, H.; Mohammadinezhad, R.; Rahmani, F. Design of a pDNA nanocarrier with ascorbic acid modified chitosan coated on superparamagnetic iron oxide nanoparticles for gene delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127743.
- Weiss, A.-V.; Fischer, T.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L.; Schneider, M. Mechanical properties of gelatin nanoparticles in dependency of crosslinking time and storage. Colloids Surf. B Biointerfaces 2019, 175, 713–720.
- Theodoropoulos, D.; Rova, A.; Smith, J.R.; Barbu, E.; Calabrese, G.; Vizirianakis, I.S.; Tsibouklis, J.; Fatouros, D.G. Towards boron neutron capture therapy: The formulation and preliminary in vitro evaluation of liposomal vehicles for the therapeutic delivery of the dequalinium salt of bis-nido-carborane. Bioorg. Med. Chem. Lett. 2013, 23, 6161–6166.
- Danışman-Kalındemirtaş, F.; Birman, H.; Karakuş, S.; Kilislioğlu, A.; Erdem-Kuruca, S. Preparation and biological evaluation of novel 5-Fluorouracil and Carmofur loaded polyethylene glycol / rosin ester nanocarriers as potential anticancer agents and ceramidase inhibitors. J. Drug Deliv. Sci. Technol. 2022, 73, 103456.
- Taheri-Kafrani, A.; Shirzadfar, H.; Abbasi Kajani, A.; Kudhair, B.K.; Jasim Mohammed, L.; Mohammadi, S.; Lotfi, F. Functionalized graphene oxide/Fe3O4 nanocomposite: A biocompatible and robust nanocarrier for targeted delivery and release of anticancer agents. J. Biotechnol. 2021, 331, 26–36.
- Carrera Espinoza, M.J.; Lin, K.-S.; Weng, M.-T.; Kunene, S.C.; Wang, S.S.-S. In vitro studies of Pluronic F127 coated magnetic silica nanocarriers for drug delivery system targeting liver cancer. Eur. Polym. J. 2021, 153, 110504.
- Kaliyamoorthi, K.; Ramasamy, S.; Pillai, A.S.; Alexander, A.; Arivarasu, A.; Enoch, I.V.M.V. Camptothecin-loaded holmium ferrite nanocarrier. Expanded activity on breast cancer cells. Mater. Lett. 2021, 285, 129164.
- Baghbanbashi, M.; Pazuki, G.; Khoee, S. One Pot Silica Nanoparticle Modification and Doxorubicin Encapsulation as pH-Responsive Nanocarriers, Applying PEG/Lysine Aqueous Two Phase System. J. Mol. Liq. 2022, 349, 118472.
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-gum arabic embedded alizarin nanocarriers inhibit biofilm formation of multispecies microorganisms. Carbohydr. Polym. 2022, 284, 118959.
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-conjugated graphene quantum dots as versatile and pH-sensitive nanocarriers for enhanced delivery of curcumin targeting to breast cancer. Mater. Sci. Eng. C 2021, 121, 111809.
- Alvarez-Román, R.; Naik, A.; Kalia, Y.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62.
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151.
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080.
- Alp, E.; Damkaci, F.; Guven, E.; Tenniswood, M. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int. J. Nanomed. 2019, 14, 1335.
- Wang, T.; Meng, Q.; Lin, L.; Yang, L.; Zhao, W.; Sun, D. Self-assembled dehydropeptide nanocarrier as a delivery system for antitumor drug temozolomide. Bioorg. Chem. 2022, 124, 105842.
- Zohreh, N.; Rastegaran, Z.; Hosseini, S.H.; Akhlaghi, M.; Istrate, C.; Busuioc, C. pH-triggered intracellular release of doxorubicin by a poly(glycidyl methacrylate)-based double-shell magnetic nanocarrier. Mater. Sci. Eng. C 2021, 118, 111498.
- Thodeti, S.; Reddy, R.; Kumar, J. Synthesis and characterization of pure and indium doped SnO2 nanoparticles by sol-gel methods. Int. J. Sci. Eng. Res 2016, 7, 310–317.
- Sawyer, L.; Grubb, D.T.; Meyers, G.F. Polymer Microscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008.
- Hansford, G.M.; Turner, S.; Degryse, P.; Shortland, A.J. High-resolution X-ray diffraction with no sample preparation. Acta Crystallogr. Sect. A Found. Adv. 2017, 73, 293–311.
- Sabbagh, H.A.K.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Abudayeh, Z.; Ayoub, R.; Abudoleh, S.M. A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers 2020, 12, 772.
- Rachmawati, H.; Yanda, Y.L.; Rahma, A.; Mase, N. Curcumin-loaded PLA nanoparticles: Formulation and physical evaluation. Sci. Pharm. 2016, 84, 191–202.
- Boddolla, S.; Thodeti, S. A review on characterization techniques of nanomaterials. Int. J. Eng. Sci. Math. 2018, 7, 169–175.
- Begum, R.; Farooqi, Z.H.; Naseem, K.; Ali, F.; Batool, M.; Xiao, J.; Irfan, A. Applications of UV/Vis spectroscopy in characterization and catalytic activity of noble metal nanoparticles fabricated in responsive polymer microgels: A review. Crit. Rev. Anal. Chem. 2018, 48, 503–516.
- Pang, S.C.; Chin, S.F.; Nadirah, A.; Tay, S.H.; Yazid, S.N.A.M. Fabrication of polysaccharide-based nanoparticles as drug delivery nanocarriers. ECS Trans. 2015, 66, 15.
- Hung, H.-S.; Bau, D.-T.; Yeh, C.-A.; Kung, M.-L. Evaluation of cellular uptake mechanisms for AuNP-collagen-Avemar nanocarrier on transformed and non-transformed cell lines. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123791.
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Effect of gelatin-stabilized copper nanoparticles on catalytic reduction of methylene blue. Nanoscale Res. Lett. 2016, 11, 438.
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning electron microscopy: Principle and applications in nanomaterials characterization. In Handbook of Materials Characterization; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–145.
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. EJH 2018, 62, 2841.
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-sensitive and hyaluronic acid-functionalized nanoparticles for improving breast cancer treatment by cytoplasmic 17α-methyltestosterone delivery. Molecules 2020, 25, 1181.
