1. Introduction
With clinical advances, the old populace is rising quickly and the world at present is confronting the “aging era”, which accompanies social issues [
1]. To cope with such a situation, metal ions have gained interest globally owing to their consequential role in a biological system facilitating enzyme function, oxygen transport, and redox chemistry as well as their role as pharmaceuticals and diagnostic agents in the field of regenerative medicine and in tissue engineering attributable to the prospect of using their novel properties for therapeutic purposes [
2]. They are engaged in intercellular and intracellular interactions, maintain osmotic pressure and electrical charges in the body, are involved in photosynthetic and electron transfer reactions, assist in pairing, stacking, and stability of nucleotide bases, play a role as cofactors for enzymes, excite chains of reactions coupled with cell signaling pathways contrary to tissue equilibrium, and engaged in the regulation of DNA transcription [
3]. They play such an important role in the functioning of nerve cells, muscle cells, the brain and heart, oxygen delivery, and other biological processes that we cannot envision life without them [
4]. The broad range of pathological conditions in which metallic ions are involved reflects these properties, which are far from precise [
5]. Metals are effectively used for load-bearing applications in the biomedical system due to their appealing mechanical properties, such as strength, stiffness, and fatigue life [
6]. Metals are tightly controlled in the natural environment owing to their reactivity, and abnormal metal ion concentrations are linked to a variety of pathological disorders, as well as cancer [
7]. In humans, metal ions are necessary for numerous significant functions [
8]. Diseases such as pernicious anemia caused by iron deficiency, growth retardation caused by inadequate dietary zinc, and heart disease in infants caused by copper deficiency extreme malfunction, metabolic disorders such as cancer, central nervous system disorders, infectious diseases, and carcinogenesis or death can all be caused by a scarceness of certain metal ions. Anomaly metallic ion metabolism, on the other hand, may lead to pathological conditions like hemochromatosis, Wilson disease, and Menkes disease [
8]. Owing to the metallic ions’ unique properties, such as Lewis acidity, hydrolytic, and redox activity, electrophilicity and valency can modify cellular activities sustaining the cell metabolism or induce lethal effects such as a minimum scarcity of certain metallic ions, and are involved in the pathogenesis of different chronic diseases like diabetes mellitus, rheumatoid arthritis, coronary heart disease, epilepsy, nephropathy, and a range of bone-related pathologies [
9].
Particular “bioinorganic” substances, such as metallic ions like copper, strontium, zinc, cobalt, silicon, and boron, have emerged as promising therapeutic drugs with the ability to boost bone formation owing to their stimulating effects on osteogenesis and angiogenesis in the past decades [
10]. Moreover, others (such as copper, zinc, and silver) have additional therapeutic properties, such as anti-inflammatory and antibiotic properties [
11]. Therefore, it is important to comprehend metal ions at the molecular level to cure ailments due to insufficient metal ion activity [
12]. As a result, monitoring the precise level as well as their role in the body will enhance the effectiveness and selectivity of metallic ions’ therapeutic effects [
13].
Furthermore, when definite metallic ions are directly absorbed, their ionic states are unstable, causing noxious effects. Immense studies have been undertaken to create matrices to control the local release of metallic ions to cope with that kind of scenario [
14]. The design of matrices for the local delivery of relatively high concentrations of metallic-ion-based drugs to target tissues with reduced systemic adverse effects is of high interest because recent metallic-ion-based drugs are vulnerable to direct severe systemic toxicity; thus, the design of matrices for the local delivery of relatively high concentrations of metallic-ion-based drugs to target tissues with reduced systemic adverse effects is of high interest [
15]. To optimize metal ion delivery for therapeutic use, the degree of metallic ion loading into matrices for local delivery, as well as the controlled and sustained release of the loaded ions, is undeniably significant [
16]. Uncontrolled metal ion release, on the other hand, may have harmful implications, as with the case of metal implant corrosion, which results in the release of large quantities of metal ions into the tissues in intimate interaction with the implant and through the systemic circulation, causing problems such as immune and inflammatory responses [
17]. Metals’ mechanical and electrical properties have led to their use throughout biomedical engineering, especially in the form of implantable medical devices [
18]. Metals are used in almost every orthopedic tool, but metal-on-metal (MoM) bearings are of particular interest due to the possibility of detrimental biochemical functions caused by the inappropriate generation of metallic particles as well as ions [
19]. Metal ions are effective tools, but further research into their interactions with living systems is required to establish the boundaries that restrict their safe and therapeutic use [
20,
21].
2. Therapeutic Metallic Ions
2.1. Gallium
Gallium (Ga) is a soft, silvery metal member of Group XIII of the periodic table. Though the element itself does not have any direct biological function in the human body, it is therapeutically beneficial for many biological processes.
2.1.1. Gallium’s Physical, Chemical, and Biological Properties
It is claimed that gallium finds its application due to its close characteristics with the iron (Fe) ion; for instance, the chemistry, ionization potential, radii, etc. [
1]. Ga does not participate in the redox reactions. Unlike Fe, it does not interfere with the oxygen uptake of heme molecules. It also has different stability [
1].
Moreover, similarities exist between Ga and Zn as well, thus another therapeutic function involves the substitution of Zn with Ga in metalloproteins to dose-dependently inhibit alkaline phosphate [
22]. Due to its compatibility Ga complexes have successfully been doped in several different types of matrices, depending on the application requirements [
23]. Recently, Ga-doped 45S5 Melt-quench-derived bioglass (BG) was reported to illustrate good biocompatibility [
24].
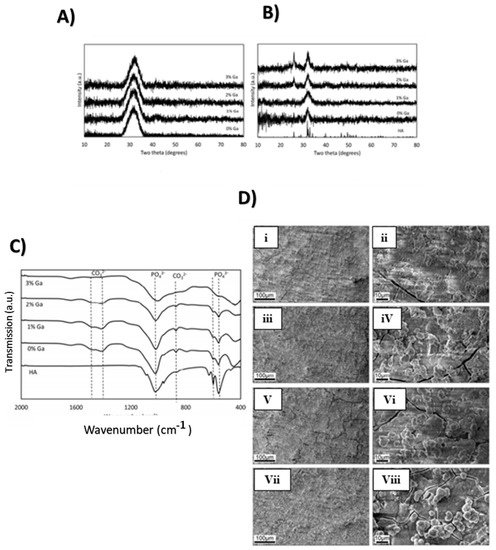
Figure 2A shows the XRD diffraction pattern of the Ga-doped BG, which indicates the amorphous nature of bioactive glass in an unreacted state.
Figure 2B shows a layer of amorphous calcium phosphate upon reacting with SBF (simulated body fluid) [
25,
26].
Figure 2C refers to Fourier transform infrared (FTIR) spectra of Ga-doped BG after being immersed in SBF for a week. Carbonate and phosphate bands appeared after immersion in SBF which is a characteristic of hydroxyapatite (HA) formation. It was evident that Ga doping did not affect the bioactivity of the BG. Furthermore, SEM (scanning electron microscopy) images after immersion in SBF with varying Ga composition are presented in
Figure 2D. All the samples showed the formation of fused spherical apatite-like crystals after being immersed in SBF for a week [
3].
Figure 2. (
A) XRD spectrum for unreacted BG; (
B) XRD spectrum for BG after 7 days in SBF, with hydroxyapatite shown as a reference; (
C) FTIR spectra of Ga doped BG following immersion for 7 days in SBF; (
D) SEM image of BG after 7 days in SBF. (Adapted from [
3]).
2.1.2. Properties and Applications
Anticancer Effects of Gallium
Ga complexes have been used for the treatment of cancer. The multiplying cells are sensitive to Ga due to their high requirement of Fe in their DNA replication, enzyme activity, respiration, and many other essential cellular processes. Due to chemical and structural similarities [
2], Ga is taken into the cells by transferrin (TF) receptors. This changes the pH and prevents uptake and utilization of Fe by the cancer cell. This inhibits DNA replication and results in apoptosis [
1]. Gallium nitrate is considered the first gallium compound that has exhibited anticancer properties in humans [
21].
Antimicrobial Activity in Gallium Compounds
Gallium nitrate complexes have usually been used for ease of fabrication [
27]. Several Ga compounds show antimicrobial activity which is therapeutically promising. Gallium nitrate is extremely effective against
P. aeruginosa in a dose-dependent manner at concentrations greater than 1 µM; at 0.5 µM biofilm growth was prevented [
11], and at 100 µM established biofilms were destroyed [
28]. Though there is limited research confirming the antiviral effect of Ga, it was effective against HIV by targeting the host RNA [
29].
Gallium Compounds as Anti-Inflammatory and Immunosuppressive Agents
Several studies involving in vitro and in vivo systems have shown that gallium compounds have immunosuppressive activity in animal models of autoimmune disease. Gallium nitrate was shown to suppress experimental autoimmune encephalomyelitis and prevent adjuvant inflammatory arthritis through suppression of macrophage function and T-cells in rat models while the anticancer effects of Ga were also studied [
30]. Pro-inflammatory T-helper type 1 cells become inactive by iron deprivation owing to Ga uptake in place of Fe which is comparatively irreducible [
31]. Transferrin-gallium and gallium nitrate were shown to inhibit the mixed lymphocyte culture response and prolong the survival of mice with severe graft-versus-host disease in a murine bone marrow transplant model [
12]. Despite these interesting preclinical observations, the immunomodulatory and anti-inflammatory properties of gallium appear to have not been investigated in rigorous clinical studies [
9]. Further investigations are required to warrant and establish whether the results of these in vitro studies are relevant to patients with inflammatory or autoimmune diseases [
10]. The potential effects of gallium on inflammation and the immune system should be kept in mind when gallium compounds are being used for the treatment of other conditions [
4].
Anti-inflammatory properties of gallium are recognized but not fully utilized in the field of prosthetics. Defensin (De hBD-1) has potent antimicrobial activity in vivo as part of the innate immune system. However, this property is not being employed successfully under in vitro conditions. Polylactic acid films were synthesized and modified with Ga and simultaneously functionalized using De. Both Ga and De independently and synergistically reduced the total number of live bacteria on the implant surface (which was coated with thin films). The surface treated with this film was able to kill bacteria and reduce inflammation compared to the untreated surface [
10,
31].
Effects of Gallium Associated with Hypercalcemia and Bone Metabolism
The anti-bone-resorptive effect of Ga influenced the study on Ga used in the treatment of hypercalcemia and other bone diseases (osteoporosis, Paget’s disease, etc.) [
5]. Ga dose-dependently inhibits the resorption of bone by osteoclasts, without being toxic to the osteoclasts (it inhibits acid production in osteoclasts) [
32]. Osteoporosis affects bone fragility due to its mass reduction, resulting in fractures. Due to its properties, including being a bone resorption inhibitor, gallium can influence osteoporosis healing [
8]. Researchers have synthesized organic gallium (OG), which is formed through a mixture of gallium and yeast [
33]. Results showed that OG may increase bone volume and bone area [
34], cortical thickness, and trabecular thickness and may decrease the number of osteoclasts in the osteoporotic invoice [
35], thus confirming that the obtained data allow the OG to heal osteoporotic fractures [
36].
Antimalarial Agent
Metalloporphyrins are potent heme-polymerization inhibitors and the central ion plays a major role in the inhibitory action of metalloporphyrins. Begum et al. [
6] evaluated the in vitro antimalarial activity of 10 different metalloporphyrins including four gallium derivatives: gallium protoporphyrin IX (GaPPIX), gallium salt protoporphyrin IV (GaPPIXNa2), gallium deuteriopropyrin (GaDPIX), and hematoxylin gallium (GaHPIX) [
37]. The results showed that all derivatives inhibited heme polymerization, however, GaPPIX and GaDPIX showed more significant results during in vitro tests, presenting IC
50 values below 80 µM in the
trophozoite form of
Plasmodium falciparum [
38].
2.2. Bismuth
Bismuth (Bi) has been used as a therapeutic agent for over two centuries in the form of various complexes [
39]. Most of the bismuth salts used nowadays are safe, having fewer side effects, most of which are quantifiable and treatable [
40]. They are used within set quantities which have proven to be most effective via research and reported experimentations [
41]. Toxicity associated with bismuth compounds is usually a result of their unsupervised use. The clinical efficacy of bismuth compounds was evaluated and the possibility of bismuth-induced toxicity is rare when supervised and used according to the specified dose [
42].
2.2.1. Properties and Applications
Among the many different biological advantages of bismuth, the most effective and commonly studied is its use in the treatment of gastrointestinal disorders [
43]. The earliest recorded use of bismuth is for the protection and healing of skin and ulcers [
44]. Other than this, bismuth salts were found to be effective in the treatment of syphilis, hypertension, dyspepsia, diarrhea,
H-pylori infection, etc. [
45,
46,
47,
48,
49,
50]. Among the various compounds of bismuth, colloidal bismuth subcitrate (CBS), bismuth subnitrate (BS) [
51,
52], bismuth subsalicylate (BSS), and ranitidine bismuth citrate (RBC) are explored in the literature for their effectiveness and mode of action as well as modifications [
53,
54,
55,
56].
Antibacterial Action
Bismuth salts exhibit antibacterial action against various gastrointestinal tract pathogens including
E-coli,
Salmonella,
Vibrio cholera, etc. [
46]. The mechanism of bactericidal action of bismuth is still unclear, but several proposed mechanisms are summarized [
41]. Complexes of bismuth with bacterial wall and periplasmic membrane have been analyzed via microscopic studies. These structural studies have shown how bismuth complexes bind to the bacterial cell wall disintegrating the
H-pylori [
41,
47].
The antimicrobial action of bismuth makes it an effective coating on titanium implants. The titanium surface is coated with a Bi-doped nanohydroxyapatite layer via a supersaturated calcification solution contaning bismuth salt. Successful coatings indicate good radiopacity, making them a good choice for dental and orthopedic fields. Moreover, the coating shows promising antimicrobial activity against
Escherichia coli [
41,
42,
45].
Anti-Leishmaniasis Property
A leishmaniasis is a group of diseases caused by protozoan parasites of the
Trypanosomatidae family and characteristically caused by the bite of an infected female sandfly [
48,
49]. The activity of nonsteroidal anti-inflammatory drugs (NSAIDs) like naproxen, mefenamic acid, ketoprofen, diflunisal, and their corresponding homoleptic tris-carboxylate Bi(III) complexes, were investigated against leishmaniasis major promastigotes and human primary fibroblast cells for 48 h. Studies showed that the activity remains significant at only the highest concentration, ~500 µg/mL against L. major parasites, however, that concentration is considered too high for practical use [
50].
Inhibition of Enzyme Activity
Bi was also used to inhibit enzyme activity, which in turn affects the organism’s growth [
51]. Adherence of pathogenic organisms to the epithelial cells on the intestinal lining is also prevented by using bismuth compounds [
52]. Synthesis of ATP (adenosine triphosphate) in
E. coli was shown to be inhibited by bismuth subsalicylate (BSS) [
53].
Peptic Ulcer Treatment
CBS has been successfully used to treat both gastric and duodenal ulcer diseases. CBS especially shows a low relapse after cessation of the treatment, which is attributed to the
H-pylori eradication [
54]. This elimination reduces the possibility of reinfection caused by the organism. It has been practically observed that the effect of CBS is even better when the compound is administered along with antibiotics [
55]. CBS and BSS are the most commonly used compounds due to their functional similarities; however, they do have different mechanisms of action [
56]. CBS is mostly used to treat peptic ulcer disease and with the addition of antibiotics is also effective against
H-pylori [
57] while BSS is mostly used for treating and preventing infective diarrhea [
58]. CBS, BSS, and RBC when used simultaneously exhibit synergistic effects for eradicating
H-pylori [
59]. Among all the bismuth compounds, RBC is relatively new and brings the added advantage of acid suppression and high compliance [
41,
42].
2.3. Magnesium
Magnesium is the fourth most abundant cation in the body and the second most intracellular cation. It is known to be essential for several enzymatic activities in many biological functions of the human body [
60]. The importance of Mg as a therapeutic ion has been explored and is reported to be vital for several types of cells owing to the interaction with phosphate ions (ATP exists in cells normally as a chelate with Mg
2+); it acts as a cofactor for many enzymes, stimulates the growth of new bone tissue, and aids the adhesion of osteoblastic cells. Other than this, Mg is an excellent coagulant used especially in cardiac applications [
61].
2.3.1. Properties and Applications
Promotion of Osteoblast Cell Proliferation and Differentiation
Due to its many biological benefits, Mg has been used as a dopant and in the form of complexes with many different matrices among which glass-ceramic (49.13 wt.% SiO
2-.68 wt.% CaO-43.19 wt.% MgO) is the most common [
62]. The preferred synthesis technique is the sol–gel method. Other than glass-ceramics, some quaternary glass systems (64% SiO
2, 26% CaO, 5% MgO, and 5% P
2O
5 in mol.%) are also used. The synergistic effect of bioactive glasses with Mg ions promotes osteoblast cell proliferation and differentiation [
63].
Mg has two main mechanisms of interaction; it can either bind to the enzyme substrate, forming a complex with which the enzyme interacts, or it binds directly to the enzyme altering its structure. All in all, its function is related to ATP utilization. Mg is present in almost all biological cells as Mg-ATP [
64]. Therefore, the inability of Mg to perform its function either due to deficiency or uncontrolled release from the scaffolds can result in the hindrance of Mg-activated functions. Mg plays a crucial role in cellular function; in the absence of proper Mg release and activation, cell proliferation can be hindered due to reduced DNA, RNA, and protein synthesis [
60]. The addition of Mg ions in scaffolds increases the bioactivity and compatibility of the system by promoting bone cell activity. Mg is mitogenic for osteoblasts in cell culture and its depletion causes cell growth inhibition [
65,
66,
67,
68].
Due to its enzymatic activity, Mg is necessary for the proper activity of DNA and RNA polymerases. Mg is an important factor in DNA repair mechanisms within the cell, including nucleotide excision repair (NER), base excision repair (BER), and mismatch repair (MMR). DNA damage occurs constantly due to chemicals, radiation, and other mutagens, and to repair it we need Mg as it is required alongside ATP for proper enzyme activity [
66].
Mg Ions as a Coagulant
An extremely interesting and recently explored use of Mg is as a coagulant. Mg ions play a crucial role in stabilizing the native conformation of coagulation factor IX (a protein produced naturally in the body which helps the blood form clots to stop bleeding). Mg ions greatly augment the biological activities of factor IX. The cation increases the affinity between factor IXa and factor VIIa, thereby increasing the catalytic efficacy of the enzyme. Approximately 10-fold less concentration of factor XIa was enough to produce the same clotting effect in the same time as in the absence of Mg ion. A similarly reduced clotting time was observed with factor IXa coupled with Mg ions, approximately three-fold faster clotting. For factor Xa, Mg ion did not have a reasonable effect on clotting time [
60,
67].
2.4. Calcium
Calcium (Ca) is an essential component of the entire skeletal structure and is one of the most abundant metals to exist in the human body. Approximately 99% of body calcium is found in bones. It forms hydroxyapatite in combination with phosphates. The movement of Ca ion in and out of the cytoplasm acts as a signal and activator for several cellular processes [
69]. The close association of Ca with bone enables Ca-doped scaffolds to promote bone cell differentiation, bone metabolism, mineralization, and osteoclast proliferation [
70]. The hydrated calcium ion takes part in many other body functions including muscle contraction, hormonal response, neurotransmitter release, blood clotting, and protein stabilization [
71].
2.4.1. Properties and Applications
Cellular Proliferation and Differentiation
The biocompatibility of Ca allows its use in different scaffold materials. It has been successfully doped in osteochondral composites, using Type-II collagen gel with HA [
72]. The compositions of Ca ions can be varied for optimal property profile (2–4 mmol, 6–8 mmol, less than 10 mmol). It should be noted that low to medium concentrations (2–8 mmol) promote cell proliferation, differentiation, and mineralization [
73], whereas higher concentrations (greater than 10 mmol) are toxic [
74]. Moreover, calcium phosphate treatment of 3D bioactive glasses has also been employed to increase cellular attachment [
75]. The latest trend in biomedicine is the use of silica gels, and calcium-doped mesoporous silica xerogels produced using the sol–gel method [
76]. Again, low concentrations promote cellular proliferation and differentiation with higher concentrations being toxic [
74,
77].
The doped mesoporous silica gels resulted in the formation of a smooth xerogel surface as indicated by the TEM analysis . ICP analysis for mesoporous silica xerogels with variable calcium compositions (m-SXCs) indicates the change in Ca, P, and Si concentration in SBF after 1, 3, and 7 days. Ca and P ions lead to the supersaturation of SBF solution around the hybrid membrane and accelerate the formation of a bioactive apatite-like layer [
78]. In the present study, the ICP results revealed that Si and Ca ions could be released from them (m-SXC) into SBF, and that m-SXC with Ca resulted in a more rapid increase of Ca and Si ion concentrations, providing a higher basic ion concentration in the SBF solution, which might be helpful to osteoblasts responses [
79]. Similarly, the morphology of osteoclasts cultured with m-SXCs for 24 h was analyzed under a light microscope. The results indicate that Ca ions in controlled concentrations neither harmed cell morphology nor affected biocompatibility [
74].
The above-mentioned study is an example of Ca’s involvement in biomaterial engineering as a therapeutic ion. Moreover, research proves the positive function of Ca within a certain composition, above which the ion turns toxic [
80]. A very common matrix and scaffold for Ca ions, with controlled release of ions is BG [
81]. Although the ability of BG to support osteogenesis has been proved, due to its biodegradable properties, it may release ions during the degradation process and the slow degradation helps to provide a controlled release of ions, thus preventing toxicity [
82].
The superior osteointegration and biocompatibility of calcium makes it a strong candidate for coatings of metallic implants. The coatings show promising clinical in vivo and in vitro results; however, there is a lot of research still needed for their clinical application. The coatings show superior antibacterial properties but need novel methods to control the coating structure and degradation rate [
81,
82].
2.5. Germanium
Germanium and its compounds have been in use for almost two decades as therapeutic ions. Germanium is found in plants, animals, vegetables, nutrients, dry fish, beans, oysters, and biomaterials documented by Schroeder and Balassa in 1967. Oral administration of Ge-132 results in uniform distribution of germanium with minimal residual concentration. It was observed that 30% of germanium is absorbed after 12 h [
83].
2.5.1. Properties and Applications
Antitumor Activity/Malignant Pathology
Ge-132 shows antitumor activity through the activation of immune system-based mechanisms involving the role of lymphocytes and macrophages [
84]. The augmentation of natural killer (NK) activity and activation of macrophages in mice when orally administrated by Ge132 was mediated by Ge-induced interferon (IFN). The administration of IFN-containing sera (blood serum) was synthesized from Ge-132-treated mice or the passive transfer of macrophages from Ge-treated mice to mice bearing pathology tumors. Ascites are caused by cancer [
85]. It is known as malignant pathology. Malignant pathology is most typical in people with subsequent cancers, such as inhibition of tumor growth in breast cancer patients [
86].
The mechanism of Ge-132’s antitumor activity includes the role of T-cells also known as T lymphocytes, a major component of the immune system. They attack and kill the host cells, resulting in the activation of other immune cells. Thus, cytokines are produced, and other cells of the immune system are influenced due to the production of circulating lymphokines [
87]. Activated macrophages were generated from resting macrophages by these lymphokine(s). The transplanted tumors were inhibited by these macrophages. Use of Ge-132 results in inhibition of tumor growth, enhanced antimetastatic effect (related to the inhibition of cancer cell motility and invasiveness), prolonged survival time, and recovery of loss of delayed-type hypersensitivity and body weight in tumor-bearing mice [
83].
Spirogermanium is an azaspiran-germanium compound that was investigated for antitumor effect in phase I/II trials. Spirogermanium was used for therapeutic need owing to its significant negative risk tolerance, and neurologic toxicity. Spirogermanium suppresses DNA, RNA, and protein synthesis and reduces cell survival after 24 h of exposure at 1.0 mg/mL. Quiescent cells appear to be even more resistant [
88]. Cytolysis is found at higher concentration levels. Ge-132, Ge sesquioxide, stimulates interferon but also NK cellular activity in spleen cells 24 h after oral administration as well as induces peritoneal macrophage activity in rats. The general toxicity of Ge is low, aside from the tetrahydride germane, and few observations on the toxicity of Ge in humans exist. Ge is not cancer-causing and even seems to inhibit cancer development and, within the type of the organic Ge compound, spirogermanium, to destroy cancer cells [
89]. Ge compounds have no mutagenic activity and should, below bound conditions, inhibit the mutagenic activity of alternative substances. High doses of Ge could end enhanced embryonic resorption. The mineralization of sponges and limpets occurs as the Ge follows the pathway of silicium at low concentrations [
90].
Raynaud’s Disease
Organic germanium enriches oxygen supply, i.e., the oxygen consumption requirement is lowered in the liver and diaphragm. Thus, the survival rate is increased under oxygen stress. Germanium results in increased oxygen supply in the body [
91]. The blood viscosity decreases with the increased oxygen supply, resulting in the maximum blood flow to all the organs at a rapid rate. Organic germanium protects against diseases that are associated with oxygen starvation, such as carbon monoxide asphyxiation/poisoning or stroke, and Raynaud’s disease conditions. The oxygenated effect of germanium results in a glowing and warm feeling [
92].
Patients with Raynaud’s disease get relief after taking organic germanium. The lattice structure of germanium contains negative oxygen ions, used as a substitute for oxygen. This results in the elimination and attraction of acidifying hydrogen ions thus detoxifying the blood. Water is formed by the transfer of electrons. The deficiency of oxygen results in the acidification of blood due to the accumulation of hydrogen ions. Organic germanium acts as the electron sink increasing the energy without increasing the oxygen supply during oxidative metabolism [
83].
Antioxidant Effects
Germanium protects against radiation. Lipid peroxidation (LPx) products, DNA hydroxylation, and protein hydroxylation products are the main biomarkers of oxidative damage. Various studies have suggested that germanium compounds show a protective effect against liver injury and have similar oxygen enriching properties and rigorously documented antioxidant effects [
93].
The cell membranes are protected against damage by free radicals using antioxidant systems such as superoxide dismutase, glutathione peroxidase, catalase, etc., and nonenzymatic (glutathione, ceruloplasmin, vitamins) systems [
92]. Natural antioxidants such as vitamins C and E exert a protective effect against chromosomal damage by reactive species generated by the irradiation. Glutathione peroxidase is an enzyme system known to have protective effects against cell damage by highly reactive oxidants [
89]. GPx activity increases for the elimination of O
2− and H
2O
2 formed by radiolysis of water [
91]. In individuals undergoing radiotherapy, the increase of inflammation results in the production of free radical species, thus inducing an increase in the activity of GPx and other antioxidant defense systems [
94].
As a Protective Agent
Ge-132 administered in radiotherapy protects cancer patients from the killing of red and white blood cells due to radiation exposure. The germanium atoms attach to the red blood cells and protect them from electrons by diverting them [
95,
96,
97]. Alpha-tocopherol protects against peroxidation damage via a free-radical-scavenging mechanism [
87]. Cysteine is known to increase the endogenous antioxidant levels by enhancing intracellular stores of glutathione. New prepared germanium L-cysteine a-tocopherol is a protective agent against gamma-irradiation-induced free radicals’ production and liver toxicity [
92].
Liver cells or hepatocytes have access to the liver’s blood supply via sinusoids, i.e., the small capillaries. Hepatocytes are involved in the production of bile, a metabolic function. Light microscopic examinations of liver sections of control animals exhibited normal constructions, while liver sections of rats exposed to gamma irradiation showed liver fibrosis and necrosis with mononuclear leucocytic inflammatory cells, infiltrating the dilated portal vein in the portal animals pretreated with germanium L-cysteine a-tocopherol, and regeneration of the hepatocytes to the normal structure. Furthermore, the microscopic structure of hepatic cells in the area is associated with the proliferation of diffuse Kupffer cells. The liver section exhibited tissue degeneration, lymphocyte infiltration, and vascular degeneration of the hepatocytes. In contrast, the irradiated group of rats treated with germanium L-cysteine a-tocopherol alone showed a normal shape like the control hepatic cells [
93].
As Biocompatible Coatings
Tungsten-germanium coatings deposited using magnetron sputtering have shown good biocompatibility and tribological properties. Biocompatibility analysis shows that the cell cultures are favorably attached to the coating surface and the antibiofilm activity of the coating is also promising against two common bacterial strains, i.e.,
S. aureus and
P. aeruginosa [
92,
93].
2.6. Chromium
Chromium (Cr) was found in 1797 and was given this name due to its color features. In nature, chromium is found as red lead ore, i.e., PbCrO
4, and chromium iron stone, i.e., FeCr
2O
4. The commercial use of chromium ironstone is very common nowadays and it is also used in metallurgical processes. It is utilized in lather tanning, paints, wood preservation, production of cement, insulin signaling, and chemicals of laboratory, etc. Chromium can cause skin allergies such as contact dermatitis [
98].
2.6.1. Properties and Applications
Diabetes Mellitus and Insulin Signaling
Chromium is widely used in insulin signaling. Gene expression, metabolism of various nutrients such as proteins, sucrose, lipids, etc., and mitogenesis are influenced by a hormone called insulin. In the insulin molecule, there are two peptide chains, i.e., A and B, with 51 amino acids and disulfide bonds [
99]. The polypeptide chain is first converted to proinsulin and then to insulin. Insulin is released via stimulation, i.e., enhanced glucose concentration results in the secretion of insulin; this change in glucose concentration acts as the primary stimulant. Insulin enhances the rate of uptake of glucose, thus maintaining and regulating glucose homeostasis. The disturbance in the insulin signaling pathway can cause a disease type 2 diabetes mellitus (T2D). The insulin first binds to the insulin membrane receptor (IR) having a and b subunits, this is termed autophosphorylation [
100]. The activation of insulin receptor tyrosine kinase (IRTK) is achieved by the binding of insulin, hence stimulating autophosphorylation. In the second step, this autophosphorylation of the insulin leads to the activation of enzymes towards IRS, i.e., intracellular insulin receptor substrate proteins [
101].
Trivalent chromium has been considered essential for humans for over 30 years. It is involved in the metabolism of lipids and proteins and the insulin signaling system. The intake of chromium in a diet. i.e., 200–1000 mg Cr/day, improves blood insulin and glucose levels [
102].
Insulin sensitivity is increased with the increased phosphorylation of the insulin receptor. The ß subunit undergoes auto-phosphorylation due to the conformational changes caused by the binding of insulin to the α subunit in the insulin receptor [
103]. The chromium moves to the insulin-dependent cells from the blood when the blood sugar level increases the insulin level. After that, the chromium attached to transferrin is transferred to apochromodulin (low molecular weight chromium attaching substance) [
104]. Apochromodulin, when it binds to 4–5 moles of chromium, becomes activated and thus, insulin receptor kinase activity is increased. The binding insulin is used to convert inactive insulin to an active form. This activates the binding of Cr to apochromodulin and the movement of Cr from transferrin into the insulin-dependent cells [
105].
The chromium in the form of chromium chloride inhibits oxidative stress and TNF-alpha (tumor necrosis factor-alpha) secretion due to the interaction of chromium with cytokines (TNF-alpha, IL-6) and the peroxidation of lipids. The antioxidative effect is important in insulin signaling to lower TNF-alpha secretion and prevent lipid peroxidation [
106]. The membrane lipid depots change due to the chromium insulin signaling action. The decrease in membrane fluidity decreases insulin-stimulated glucose transport. Chromium increases membrane fluidity in the presence of insulin [
107].
Anticarcinogenic Effect
The cellular oxidative damage is caused by hexavalent chromium (Cr(VI)) which is highly reactive [
108]. Reactive oxygen species (ROS) are generated, which have high reactivity, a short span of life, and oxygen-containing species, i.e., O
2•, H
2O
2, and
•OH. The excessive production of ROS results in oxidative damage mainly in cells and tissues. Cr(VI) is reduced to its lower oxidation state, i.e., to Cr(V). The spectrum of ROS is generated by Cr(VI). Antioxidants are used to prevent oxidative damage. However, when more prooxidants exist then oxidative cell damage by chromium can happen. ROS can be generated directly during the reduction of Cr(VI).
In general, there are two pathways in the mechanism of Cr(VI)-mediated ROS generation. Cr(VI) can directly generate ROS during its reduction and subsequent reaction with cellular small molecules such as glutathione (GSH) and H
2O
2 [
109].
Glutathione-derived thionyl radical (GS•) is generated by the reaction of Cr(VI) with GSH. The GS• generation increases with the increase in GSH concentration.
H
2O
2 is formed due to the generation of radicals O
2•− by dismutation reaction. The reduction of Cr(VI) to Cr(V) results in the generation of oxygen radicals. The
•OH radicals are produced by the reaction of Cr(V) or Cr(IV) with H
2O
2.
Thus, Cr(VI) can be reduced to Cr(V) [
109]. Oxidative DNA damage is caused by the genotoxic activity of carcinogenic Cr(VI) compounds. However, stable Cr-DNA binding is attained by the reduction of Cr(VI). This results in a decrease in electrophoretic mobility of supercoiled DNA as shown in
Figure 7B. The unwinding of supercoiled plasmid DNA occurs at a high concentration of Cr(VI) and thus, they comigrate with relaxed DNA molecules. The DNA molecules containing Cr atoms show decreased staining with DNA dye ethidium bromide. Ethidium bromide fluorescence was recorded for the chromium ions involved in Cr-DNA binding.
2.7. Lithium
2.7.1. Properties and Applications
Manic Depression Treatment/Bipolar Disorder
Patients with bipolar disorders utilize lithium ions as these ions are effective in overcoming this disorder. Lithium salts are used for the treatment of this disease [
114,
115]. The impact of lithium on intracellular neurotransmission results in the normothermic action of Li; the main area of this particular action is the central nervous system. Voltage-dependent sodium channels working on the principle of concentration gradient are used by lithium for the penetration in the interior of the cell via diffusion mechanism [
116]. The permeability of lithium ion is similar to sodium ions. Thus, they can easily pass through these channels. The ionic radius of anhydrous lithium is the same as anhydrous magnesium, but less than the radius of sodium. The concentration of lithium is more in extracellular fluid than in intracellular fluid due to the use of sodium–lithium countertransport (SLC) for its displacement from the cell. The therapeutic effect of lithium for the treatment of mental alterations is highly impacted by the regulation of the lithium clearance rate. The SLC mechanism is not appropriate for the treatment of affective disorders [
117]. The action of lithium becomes interdependent with the function of various vitamins, hormones, and enzymes and multi-factorial by incorporating biochemical mechanisms. The action of lithium ions in cells is dependent on their competition with Na
+ and Mg
2+ ions due to the similarity in their atomic radius [
118]. The inhibition of enzymes and dependence on Na
+ and Mg
2+ ions are responsible for the therapeutic effect of lithium. The therapeutic effect of lithium is utilized in intracellular processes and nerve transmission pathways [
119].
Lithium can be incorporated into bioactive glasses (BGs). The most common are silicon-based LiBG, lithium phosphate bioglass (LiPBG), and lithium borate bioglass (LiBBG) [
120]. The LiPBG and LiBBG release lithium ions at a faster rate as compared to silicon-based LiBG as it is particle size-dependent [
121,
122,
123,
124,
125,
126,
127].
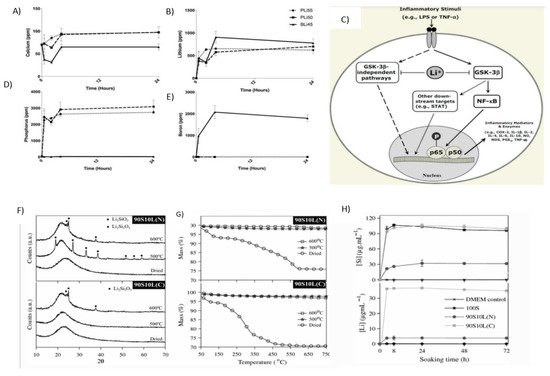
Figure 8A–D shows that after 4 h, the concentration of lithium in the cell is more than 500 ppm and remains stable for up to 24 h. At the same concentration, i.e., 6mg/mL, LiPBG and LiBBG release lithium ions at a faster rate as compared to silicon-based LiBG. Silicon-based LiBG releases 300 ppm Li [
122,
123].
Figure 8. (
A–
D) shows that in 4 h, the concentration of lithium in the cell is more than 500 ppm and remains stable up to 24 h; elemental analysis results are shown (
A) Ca, (
B) Li, (
C) P, and (
D) B after the soaking of 6mg/mL LiPBG and LiBBG for 24 h. Adapted from [
122]. Reproduced with permission from Dental Materials. (
E) shows the association between lithium and inflammation. Adapted from [
124]. Reproduced with permission from Springer. (
F) shows XRD patterns. (
G) shows the TGA results for thermal stabilization of sol–gel glasses at 500 and 600 °C, and (
H) shows the concentration profiles of lithium and silicon immersed in the DMEM. Adapted from [
127]. Reproduced with permission from Springer.
Anti-Inflammatory Agent
Lithium acts as an anti-inflammatory agent. Glycogen synthase kinase-3β GSK-3β results in enhanced inflammation in mice by facilitating the activity of transcription factor and nuclear factor (NF)-Κb [
123]. The anti-inflammatory effect of lithium associated with GSK-3β inhibition is not only due to the inactivation of NF-κB; STAT (signal transducer and activator of transcription) activation reduction also results in an anti-inflammatory effect.
Figure 8E shows the association between lithium and inflammation [
124].
The SiO
2-Li
2O glass was shown to be synthesized by the sol–gel process. Lithium nitrate (90S10L(N)) or lithium citrate (90S10L(C)) was used as a precursor of lithium [
125].
Figure 8F illustrates the X-ray powder diffraction (XRD) pattern for the SiO
2-Li
2O glass synthesized from lithium citrate and lithium nitrate. Furthermore, the effect of heat treatment at 500 °C and 600 °C was studied. It was observed that the Li ion was successfully doped by using both the precursors. The lithium was delivered at a therapeutic level and proved successful for cartilage repair [
126]. The response of chondrocyte cells responsible for the cartilage production to the glass was observed. The stabilization parameters are set for the doping of lithium ions in the silica network.
Figure 8G is showing the results of thermogravimetric analysis (TGA) and X-ray powder diffraction (XRD) with the increment of 50 °C from 400 °C to 650 °C [
127].
90S10L(C) and 90S10L(N) are immersed in Dulbecco’s Modified Eagle Medium (DMEM) without cells, and the successful release of lithium and silicon ions is observed. The results of changes in concentration are tabulated and analyzed after 3 days of immersion.
Figure 8H shows the concentration profiles of lithium and silicon immersed in the DMEM [
127].
Wound Healing/Anticoagulating Agent
Lithium plays a significant role in preventing blood clotting and thus, promotes wound healing. The pathway factors are prothrombin and fibrin stabilizing factors. The carboxylation of the clotting factor is caused by the reduced form of vitamin K [
128]. The clotting factor gets a negative charge by the addition of carbon dioxide, i.e., carboxylation. The positively charged lithium ions attract the negatively charged clotting factors and platelets. In this manner, the process of coagulation is completed [
129,
130].
The alkali-treated titanium was immersed in lithium chloride, then treated in Teflon containers. The research proposed that an increase in the concentration of lithium in bioactive glass led to a prominent decrease in bacterial activity. 58S bioactive glass with 5 mol.% Li2O substitutions for CaO was considered a biomaterial in bone repair with enhanced biocompatibility [
120,
122].
Schizophrenic Disorders
Lithium should only be used to treat schizophrenic disorders as some antipsychotics have failed; it has limited efficacy when it is used solely. The observations of various research trials on the efficiency of merging lithium with antipsychotic therapy in the treatment of schizophrenic disorders also differed [
131].
Major Depressive Disorder
Whenever antidepressant therapy does not wholly relieve the symptoms of major depressive disorder (MDD), a second augmentation entity might be incorporated into the therapy. Since the FDA has also not endorsed lithium to be used as an augmentation agent for any antidepressant for treatment of MDD, it’s has been recommended for such a purpose since the 1980s and is among the few antidepressant augmentation agents to exemplify efficacy in treating MDD in multiple controlled studies [
121]. The disorder is defined by both a pervasive and persistent depressed mood, as well as low self-esteem and a feeling of worthlessness in normally pleasurable activities. In contrast to certain other minor symptoms, the disease causes somatic symptoms such as decreased appetite (and therefore also weight fluctuations), fatigue, sleep disturbance, decreased libido, motor retardation, and bowel disturbance. Patients suffering from this major depressive disorder are at risk of developing suicidal thoughts [
132,
133].
2.8. Potassium
Potassium is utilized for the regulation of cellular electrolyte metabolism, nutrient transportation, cell signaling, and analysis of enzymes.
2.8.1. Properties and Applications
Cellular Electrolyte Metabolism
Potassium can maintain the electrolyte balance of living organisms like sodium and chloride ions [
134,
135].
Cell Signaling
Potassium is involved in cell functioning. Na
+-K
+-ATPase is present in almost all cells. It pumps potassium ions into the cell and sodium ions out of the cell; hence, a potassium ion gradient is formed around the cell membrane [
136,
137]. The potential difference is generated that is crucial for the functioning of the cell mainly for muscles and nerves. The overall potassium ion content is maintained in the body. Moreover, the potassium ions are properly distributed in the body [
134].
Diuretic Agent
Potassium is used to control blood pressure. Potassium ions in the body trigger the heart to squeeze blood. Potassium acts as a diuretic agent, hence, it decreases blood pressure by reducing extracellular fluid volume.
Figure 9B shows the lowering of systolic and diastolic blood pressure by 5.9 and 3.4 mmHg, respectively, due to the potassium intake [
136].
Nerve Functioning
Potassium plays an important role in nerve functioning. Potassium finds a sodium–potassium exchange across the cell membrane. This results in the conduction of nerve cells. Extra potassium is pumped by the cell into the interior. The creation of active impulses in neurons occurs when these ions pass through the channels in nerve cells and return to their original position.
There are wire-like extensions in the nerve cells named axons; the pulses are carried from one cell to the other by axons. Nerve axons have two key regions, i.e., the initial segment from where the impulse starts and nodes where the impulse is received. The nerve impulse starts with the movement of sodium ions into the cell. Then, in response to this, potassium channels open and permit the potassium ions’ movement [
137,
138,
139].
Brushes Coated on Artificial Implants
To prevent biofilm formation, it is common to graft anti-biofouling polymer brushes on the implant’s surface. However, these brushes can be difficult to apply. On the contrary, poly (3-sulfopropyl methacrylate potassium) (PSPMAK) brushes grafted on silicon substrates via multiple single bonds (M-PSPMAK) are a promising alternative. These brushes are more stable and remain attached to the implant longer than other available options [
138,
139].
2.9. Strontium
Strontium Sr
2+ belongs to the alkaline earth family and contains nonradioactive properties. It was discovered in 1808. Due to the rapid oxidation of Sr to form Sr
2+, it is rare to find Sr in nascent form. It is a soft silvery metal, highly reactive in water, and can bind with different proteins [
140]. Due to these properties, it is involved in different processes to form chelates and complexes. Sr
2+ and Ca
2+ have similar properties in the physiological environment as they both belong to the alkaline earth series and strontium is involved in various mechanisms of bone binding as an alternative to Ca
2+. Due to similar properties between Sr
2+ and Ca
2+, strontium participates in ion exchange with calcium [
140]. Various anionic compounds bind with strontium depending on the preference; some prefer to bind with calcium while others prefer to bind with strontium. For example, alginates prefer to bind 1.5–4.3-fold times with Sr
2+ than Ca
2+. Similarly, Ca
2+ prefers to bind with collagen and is involved in manipulating anions [
140].
According to research, 6.25 atomic percentage (at %) is reported to increase the b one area ratio and BIC in porous implants. The study also shows that this optimal concentration gives Sr-HA a closer chemical resemblance to the stoichiometric HA [
140,
141,
142].
2.9.1. Properties and Applications
Treatment of Cancer
Strontium is also utilized for the treatment of prostate cancer and bone cancer. At low concentrations, Sr
2+ is beneficial, assists in bone binding, and enhances osteoblast cell proliferation [
141]. However, at a high dose, bone resorption and bone density decrease [
142] which leads to osteomalacia, a disease generated due to the collection of the un-mineralized matrix in the skeleton [
143]. Thus, Sr
2+ is not equivalent to strontium due to these drawbacks.
Osteoporosis Treatment
Furthermore, scientists are employing strontium ranelate to analyze the treatment of osteoporosis [
144]. Drugs incorporated with sodium ranelate assist in bone growth and bone density, and inhibit osteoclast cells. Strontium ranelate consists of ranelic acid which contains dual action bone agent (DABA) and provides favorable bone resorption and harmless effects [
145]. Sr shows a beneficial response in various parts of the human body. Mesoporous bioactive glass (MBG)-doped Sr
2+ nanoparticles were synthesized using a sol–gel process. MBG provided a bioactive response and excellent biocompatibility when it was utilized with doped Sr nanobiocements compared to Sr-free. Due to the fast degradation and intriguing role of Sr
2+, it assists in achieving bioactivity. Larger radii of Sr
2+ than Ca
2+ expand the glass network and high odontogenic potential can be acquired [
146].
Osteogenic Response
The modified Stöber method is solely used to synthesize mesoporous bioactive glass nanoparticles (MBGNs). As Sr provides a bioactivity and osteogenesis, it is incorporated into different materials to acquire properties. Similarly, Naruphontjirakul et al. [
147] studied Sr-doped MBGNs with two different compositions of 6 mol.% and 14 mol.% Sr. The osteoblast cells were not affected up to the concentration of 250 µg/mL. An in vitro investigation was carried out in preosteoblast cell line MC3T3-E1 to study osteogenic response. Total cation content arises, in addition to Ca, by incorporating Sr in MBGNs which ultimately increases the dissolution rate without affecting the shape and size of particles. It was observed that Sr can enhance alkaline phosphate (ALP) activity and osteogenesis without incorporating osteogenic supplements. Thus, Sr-MBGNs assist in bone regeneration application promoting cell proliferation [
147,
148]. Furthermore, the incorporation of Sr in hydroxyapatite (HA) was shown to improve the bioactivity of HA [
149,
150].
According to a review, several studies show that strontium-coated titanium implants show higher bone-to-implant contact (BIC) and simultaneously increased the mechanical strength of the implant [
150].
This entry is adapted from the peer-reviewed paper 10.3390/prosthesis4020026